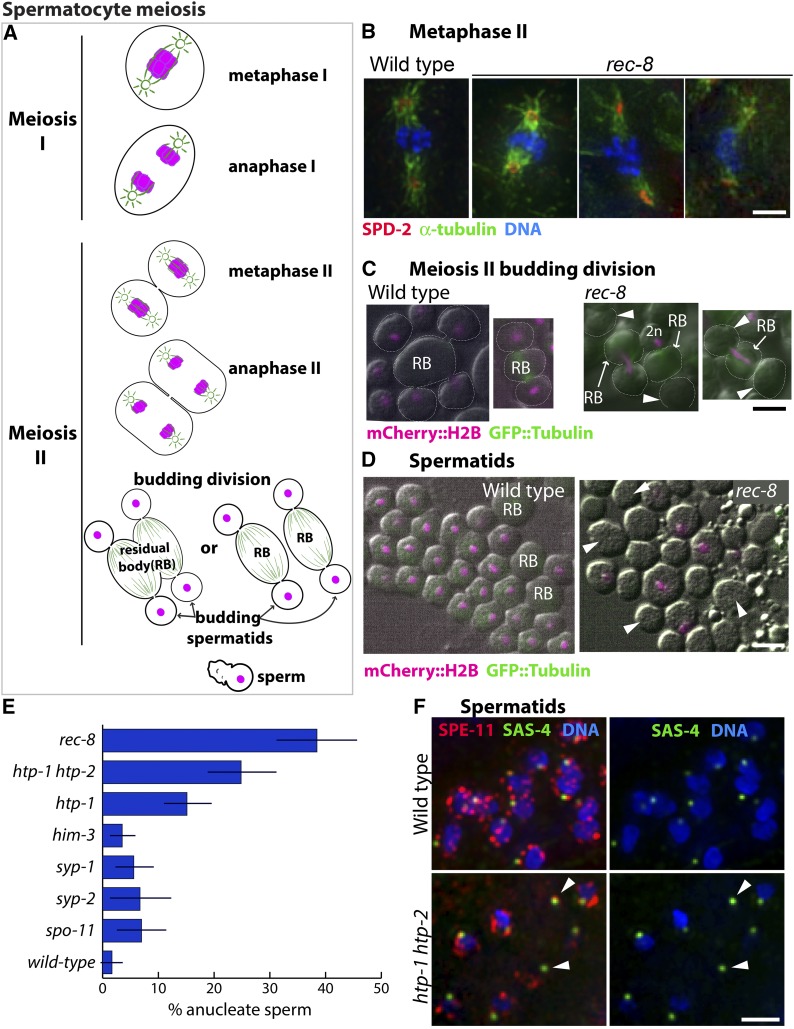
Figure 2.
Consequences of impaired meiotic cohesin function during spermatocyte meiotic divisions. (A) Diagram of the C. elegans spermatocyte meiotic divisions; microtubules are indicated in green and chromosomes in magenta. In the first meiotic division, homologs segregate to opposite poles of a spindle that contains centrosomes at its poles. This yields two secondary spermatocytes each of which undergoes meiosis II, giving rise to four haploid spermatids by a specialized budding division. Spermatids bud off and discard ribosomes, microtubules, actin, and other cellular components into the residual body (RB). Spermatids later mature into motile sperm. (B) Images of meiosis II spindles in wild-type and rec-8 mutant secondary spermatocytes, immunostained with anti-SPD-2 and antitubulin antibodies (to highlight centrosomes and spindles) and counterstained with Hoechst to visualize DNA; images are maximum intensity projections of z-stacks encompassing full spindles. rec-8 mutant spermatocytes form bipolar meiosis II spindles but chromosome segregation is impaired. (C) Live images of wild-type (AV740) and rec-8 (AV739) secondary spermatocytes undergoing the budding divisions. Contours of dividing spermatocytes, visualized by DIC, are indicated by white dotted lines; mCherry::histone H2B (shown in magenta) was used to visualize the chromosomes; arrowheads indicate anucleate spermatids. In the wild type, all chromosomes are partitioned into the budding spermatids, with each spermatid receiving a haploid (1n) chromosome complement. In rec-8 mutant spermatocytes, the second meiotic division is abnormal because all sister chromatids are already separated. Three kinds of abnormal budding divisions are shown: right, a division in which all of the DNA was retained in the residual body and two anucleate spermatids were produced; middle, a division yielding one anucleate spermatid and one (presumably 2n) spermatid containing all of the DNA; left, a division in which the chromosomes were partitioned between the residual body and one of the two spermatids, leaving the other spermatid anucleate. (D) Live DIC images of wild-type (AV740) and rec-8 mutant (AV739) spermatids and sperm expressing mCherry::histone H2B (magenta). Arrowheads mark examples of anucleate spermatids and sperm in a rec-8 mutant. (E) Quantification of the proportion of anucleate spermatids/sperm in wild type and several meiosis mutants. Error bars indicate standard deviation. Whereas wild-type sperm rarely have anucleate spermatids or sperm, a quarter or more of the spermatids/sperm produced by htp-1 htp-2 and rec-8 mutants are anucleate. The htp-1 single mutant produces fewer anucleate sperm than the htp-1 htp-2 double mutant, consistent with its less severe defect in meiotic cohesion function (Martinez-Perez et al. 2008; Severson et al. 2009). Fisher’s exact test indicates that htp-1, htp-1 htp-2, and rec-8 mutants have a significantly higher incidence of anucleate sperm (P < 0.0001) when compared either to wild type or to spo-11, him-3, syp-1, and syp-2 mutants (in which sister chromatid cohesin is retained until the second meiotic division). (F) Images of spermatids and sperm from wild type and htp-1 htp-2 mutant males, immunostained for centriolar protein SAS-4 and perinuclear sperm protein SPE-11 and stained with Hoechst to visualize DNA. Arrowheads mark centrioles and SPE-11 staining in anucleate sperm.