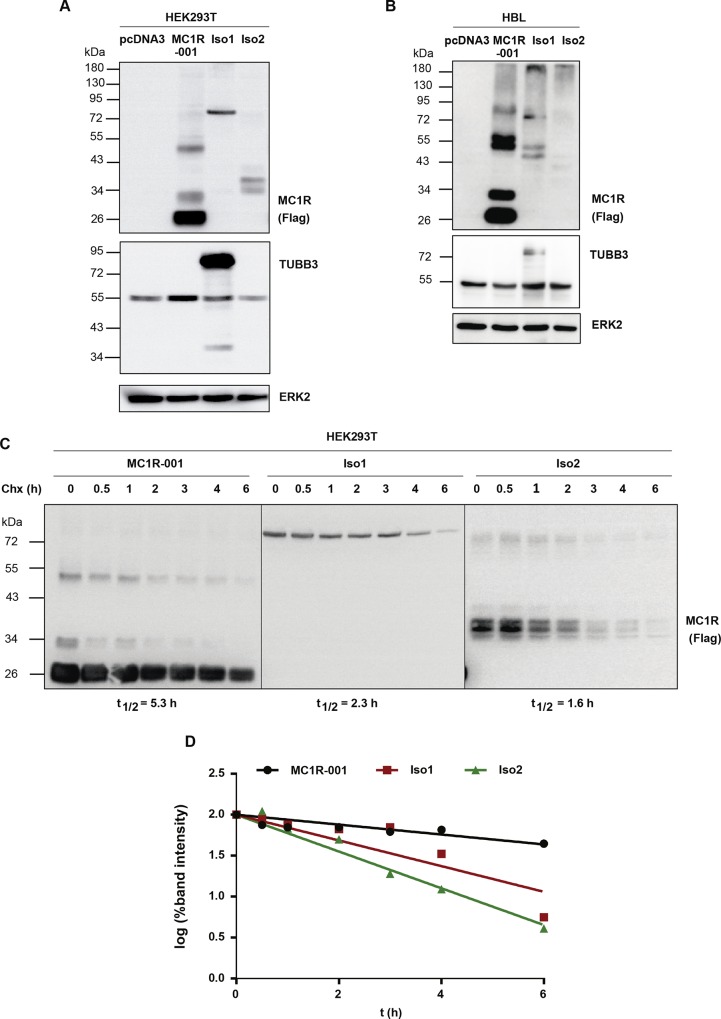
Fig 2. Electrophoretic analysis and intracellular stability of MC1R-TUBB3 isoforms.
(A) Expression of canonical and chimeric MC1R proteins in heterologous HEK293T cells. HEK293T cells were transiently transfected to express Flag-labelled WT MC1R-001, Iso1 and Iso2. Cells were detergent-solubilized, electrophoresed and blotted. For MC1R detection, cell lysates were probed with an anti-Flag monoclonal antibody (upper blot). Membranes were also probed for TUBB3 (middle blot) and ERK2 (lower blot), as loading control (n = 5, representative blots are shown). (B) Electrophoretic pattern of MC1R-TUBB3 transcripts expressed in HBL human melanoma cells. Representative immunoblots for MC1R, TUBB3 and ERK2 are shown as in panel A (n = 5, representative blots are shown). (C) Intracellular stability of MC1R-TUBB3 chimeric fusion proteins in HEK293T cells. Flag-labelled MC1R-001, Iso1 and Iso2 were expressed in HEK293T cells. Cells were incubated with the protein synthesis inhibitor cycloheximide (Chx, 0.1 mM) for the times indicated, lysed and the levels of residual proteins in cell extracts were detected by Western blot. Representative immunoblots probed for MC1R-001, Iso1 or Iso2 with anti-Flag are shown. (D) Semi-log graph for calculation of half-lives. The intensity of receptor bands in the blots as in panel C was quantitated with ImageJ and the semi-log of residual signals was plotted against time. Half-life (t½) values correspond to the slope of the resulting lines.