Abstract
OBJECTIVE
Patients with sarcomatoid renal cell carcinoma (sRCC) are known to have poor prognosis and response to systemic therapy.
We set out to examine the influence of pathological tumour characteristics on survival to aid prognostication and clinical trial design.
PATIENTS AND METHODS
A single-centre database was reviewed to identify all patients with sRCC.
Clinical variables and pathological information, including histology, necrosis, percentage of sarcomatoid features (PSF) and microvascular invasion (MVI), were recorded and correlated to outcome.
RESULTS
Analyses of 104 patients with sRCC found that the median (range) size of tumours was 9.5 cm (2.5–30), 65% of patients had areas of clear cell histology, and 69.2% had metastatic disease at presentation.
The PSF did not influence tumour size, stage, necrosis, MVI, nodes or metastasis.
A total of 85 patients (81.7%) died during the follow-up period with a median (95% confidence interval [CI]) survival of 5.9 months (4.7–8.9).
In the overall cohort, Eastern Cooperative Group performance status (ECOGPS), tumour size and metastatic disease were independent predictors of poor survival. MVI, PSF and percentage necrosis were strongly associated with outcome but were not independent predictors of outcome.
A multivariate risk model was established that incorporated six covariates (tumour size, MVI, ECOGPS, PSF, necrosis, and metastatic disease) to produce a predictive tool.
CONCLUSIONS
Both patients with localized and metastatic sRCC have very poor survival outcomes.
Pathological features MVI, PSF and necrosis are important predictors of survival and could be used in a prognostic model while grade and histology do not influence prognosis.
A prognostic model, if validated, could aid in patient counselling and/or clinical trial design.
Keywords: sarcomatoid, kidney cancer, adjuvant, prognosis, RCC
INTRODUCTION
Every year ≈ 13,000 men and women die from kidney cancer in the United States [1]. Extensive research has advanced the understanding of RCC from the classic term ‘hypernephroma’ to half a dozen distinct subtypes that have had the mechanisms of malignant transformation characterized. One entity, sarcomatoid RCC (sRCC), is poorly understood and updated classification schemes no longer consider it a distinct subtype [2,3]. These tumours generally coexist with a variable amount of carcinoma (any subtypes) and are generally believed to represent a common pathway of de-differentiation of renal tumours [4]. While only 5% of patients with RCC have sarcomatoid features, these tumours account for 15–20% of patients with advanced disease [5,6].
Improved understanding of the biology of RCC has led to the introduction of six new systemic therapies since 2005. However, agents such as sunitinib, sorafenib and temsirolimus appear to have only limited efficacy in treating patients with sRCC [7–9]. Recently, several clinical trials have specifically targeted patients with sRCC, some of which have stratified patients based on histological features, including clear-cell vs non-clear-cell histology, and by the percentage of sarcomatoid features (PSF) in the primary tumour. Stratification in such trials could help to assess therapeutic response if the histology and the PSF influences the disease biology [10]. However, currently the role of these pathological characteristics in determining biology and outcome is unclear.
Clinicians recognize that sarcomatoid tumours are associated with poor outcome, but there are a subset of patients who have exhibited extended survival. Only a limited number of studies have attempted to correlate pathological features with prognosis [5,11]. To better counsel patients with sRCC and to risk-stratify those involved in future clinical trials, we set out to determine the importance of pathological characteristics in patients in a large single-centre cohort of consecutive patients diagnosed with sRCC.
MATERIALS AND METHODS
From 1989 to 2009, all patients undergoing surgical management of suspected RCC at the University of California, Los Angeles (UCLA) were entered into a prospective database of clinicopathological variables. Tumours that were previously reported to have either sarcomatoid histology or a mention of ‘spindle cells’ in the pathology report were re-reviewed by an expert genitourinary pathologist (JWS). Tumours were designated as having sarcomatoid features using the World Health Organization classification system [12]. All clinical information and staging variables (based on 2002 TNM staging criteria) for patients were recorded at the time of surgical management. Pathological information, including histological subtype, grade of an identifiable carcinoma component, the presence or absence of microvascular invasion (MVI), the presence and percentage of necrosis, the PSF and the histological sarcomatoid pattern was recorded on re-review. Designation of the PSF and pattern of sarcomatoid elements has been described previously [6,11]. The few cases with no recognizable carcinoma element were considered unclassified and rare cases of complete sarcomatoid change were stained for epithelial markers to distinguish them from renal sarcomas.
The incidence of clinical and pathological variables was determined for the overall cohort. The influence of biological characteristics and the PSF on clinicopathological variables was characterized. Comparisons of continuous variables between subgroups of patients were made using Students’ t-test for two groups and ANOVA for multiple groups. For categorical variables, the chi-squared test was used to compare proportions between subgroups.
Overall survival of these patients was assessed from the date of nephrectomy to either their recorded date of death or censored if alive at last contact. The cohorts’ survival was calculated by the Kaplan–Meier method. The influence of pathological variables (percentage necrosis, PSF and MVI) on survival was evaluated. Univariate and multivariate Cox regression models were performed to determine the influence of clinocopathological variables on survival. P < 0.05 was considered to indicate statistical significance. Only significant variables were considered in the multivariate model. Statistical analyses were performed with R 2.9.2.
A leave-one-out cross-validation (LOOCV) receiver operating characteristic (ROC) analysis was performed to evaluate the extent to which one could predict survival using the risk score generated from the multivariate Cox model. For each training set obtained by omitting one patient, a multivariate Cox model was fitted with the covariates significant at the 0.05 level in the univariate analysis. The risk score of the test patient was obtained which equalled the weighted sum of the covariates values with the weights equal to the associated regression coefficients estimated from the multivariate Cox model. After completing the cross-validation, an ROC analysis was performed to correlate the cross-validated risk score to survival at 1, 3 and 5 years after surgery. In the ROC analysis, sensitivity was defined as the probability of correctly classifying a patient as dead, and specificity was defined as the probability of correctly classifying a patient as alive. Area under the curve (AUC) was used to measure the predictive ability of the risk score. Permutation testing was performed to evaluate the significance of the AUC. For each random permutation of survival to covariates, the LOOCV ROC analysis was performed and AUC was estimated from the permuted data. The permutation was repeated 1000 times to estimate the distribution of AUC under the null hypothesis that the survival outcome is independent of covariates. The proportion of this null distribution beyond the AUC estimated from the actual data was taken as the P value.
To assess if the cohort of the present study was generalizable to all patients with sRCC, the clinical characteristics of patients included in the Surveillance, Epidemiology, and End Results-17 (SEER-17) database were reviewed. While sRCC is no longer considered a distinct histological subtype, this feature has been routinely coded as such in the SEER-17 database since 2001. From 2002 to 2007, all patients listed as having cancer of the kidney and renal pelvis were evaluated and those with International Classification of Diseases for Oncology, 3rd edition (ICD-O-3) histology codes matching RCC (3140, 8260, 8270, 8290, 8310, 8312, 8316, 8317, 8318, 8319, 8320) were selected. Patients coded as 8318 (RCC, sarcomatoid) had all clinical characteristics evaluated, including tumour size and SEER stage (localized, regional and distant). Cancer-specific survival and life tables were generated using SEER*stat (version 6.6.2).
RESULTS
A total of 1925 patients underwent nephrectomy during this period. A total of 104 patients (5.4%) had confirmed sarcomatoid histology. Most patients had large tumour size (mean 10.7 cm), advanced tumour stage 80.5% (≥ T3) and metastases on presentation (69.2%) (Table 1). Most patients were symptomatic (88.2%), but most (88.5%) had good performance status (0/1) at time of nephrectomy. Most tumours had a recognizable carcinoma component (94.2%) and necrosis (96.1%). Most cases demonstrated clear-cell components (65.0%) (Table 2); however, all subtypes were observed. The PSF varied widely (range 2–100%) and 50% of patients had MVI.
TABLE 1.
Patient and tumour characteristics of patients with sRCC
| Characteristic | N (%) (unless noted otherwise) |
|---|---|
| Patients | 104 |
| Age, years | |
| Mean | 60.1 |
| Median | 59.0 |
| Sex | |
| Male | 74 (71.8) |
| Female | 29 (34.9) |
| Side | |
| Right | 54 (53.5) |
| Left | 47 (45.0) |
| Symptomatic | |
| Yes | 90 (88.2) |
| No | 12 (11.8) |
| ECOGPS | |
| 0 | 28 (29.2) |
| 1 | 57 (59.4) |
| 2 | 11 (11.5) |
| Tumour size, cm | |
| Mean | 10.7 |
| Median | 9.5 |
| Metastasis | |
| M0 | 32 (30.8) |
| M1 | 72 (69.2) |
| T stage | |
| T1 | 5 (4.9) |
| T2 | 15 (14.6) |
| T3a | 39 (37.9) |
| T3b | 21 (20.4) |
| T3c | 2 (1.9) |
| T4 | 21 (20.4) |
TABLE 2.
Pathological tumour characteristics
| Characteristic | N (%) unless noted otherwise |
|---|---|
| Histology | |
| Clear | 67 (65.0) |
| Papillary type I | 4 (3.9) |
| Papillary type II | 10 (9.7) |
| Chromophobe | 11 (10.7) |
| Unclassified | 10 (9.7) |
| Collecting duct | 1 (1.0) |
| Necrosis | |
| No | 4 (3.9) |
| Yes | 99 (96.1) |
| Percentage necrosis, % | |
| Mean | 27.1 |
| Median | 25.0 |
| Range | 0–75 |
| Microvascular invasion | |
| No | 50 (50.0) |
| Yes | 50 (50.0) |
| Percentage sarcomatoid, % | |
| Mean | 50.9 |
| Median | 50.0 |
| Range | 2–100 |
| Carcinoma | |
| No | 6 (5.8) |
| Yes | 98 (94.2) |
| Carcinoma grade | |
| 2 | 12 (12.5) |
| 3 | 33 (34.4) |
| 4 | 51 (53.1) |
| Sarcomatoid pattern | |
| Storiform | 4 (3.9) |
| MFH | 25 (24.5) |
| NOS | 70 (68.6) |
| Other | 3 (2.9) |
MFH, malignant fibrohistiocytoma; NOS, not otherwise specified.
Associations of pathological variables with tumour size and the presence of metastasis were investigated. The presence of MVI was correlated with tumour size. Tumours with and without MVI had mean (SD) tumour sizes of 11.6 (4.9) and 9.8 (4.2) cm, respectively (P = 0.048). There was no association observed with size PSF, histology or tumour grade (data not shown). MVI and the PSF did not correlate with the presence of metastases (data not shown). Carcinoma histology (clear cell) and higher Fuhrman grade had an increased incidence of metastatic disease; however, they failed to reach significance (P = 0.17 and 0.16, respectively).
The PSF (by quartile) and the influence of clinicopathological variables are shown in Table 3. There was no association between PSF and tumour size, MVI, lymph node involvement, percentage necrosis and metastatic spread. Tumours with increased PSF were more frequently in an advanced stage, but this failed to reach significance (P = 0.10). Increased PSF was associated with worse performance status (P = 0.03).
TABLE 3.
Percentage sarcomatoid features by quartile and clinicopathological variables
| Percentage sarcomatoid | |||||
|---|---|---|---|---|---|
| < 25% | 25–50% | 50–75% | ≥ 75% | ||
| Category | (n = 28) | (n = 16) | (n = 29) | (n = 31) | P value |
| Size, cm | 9.84 (4.02) | 10.57 (4.33) | 11.36 (5.89) | 10.72 (3.93) | 0.32 |
| Stage | |||||
| T1/2 | 6 | 2 | 3 | 9 | 0.1 |
| T3 | 19 | 12 | 20 | 12 | |
| T4 | 3 | 2 | 6 | 10 | |
| Node | |||||
| 0 | 20 | 7 | 20 | 24 | 0.12 |
| 1+ | 8 | 9 | 9 | 7 | |
| MVI | |||||
| No | 15 | 5 | 14 | 20 | 0.18 |
| Yes | 13 | 11 | 15 | 11 | |
| Percentage necrosis | 24.44 (15.53) | 30.00 (19.24) | 24.14 (12.33) | 30.81 (17.47) | 0.27 |
| Metastasis | |||||
| No | 7 | 4 | 10 | 11 | 0.75 |
| Yes | 21 | 12 | 19 | 20 | |
| ECOGPS | |||||
| 0 | 15 | 3 | 5 | 6 | 0.03 |
| 1 | 11 | 8 | 18 | 21 | |
| 2 | 1 | 3 | 4 | 3 | |
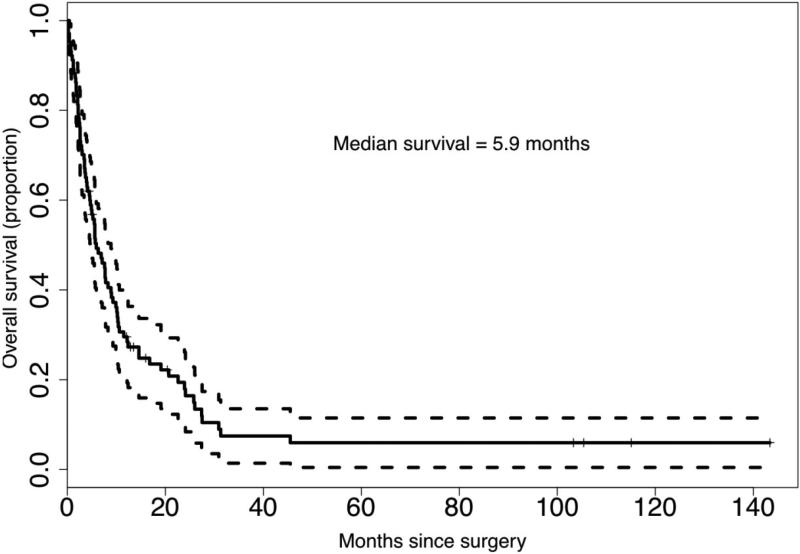
During the follow-up period, 85 patients (81.2%) died and the median follow-up of surviving patients was 12.1 months. The median (95% CI) survival of the cohort was 5.9 months (4.7–8.9) (Fig. 1). The 1-, 2- and 5-year overall survival rates for the cohort were 29.6%, 17.9% and 6.0%, respectively. The 1-, 2- and 5-year overall survival rates for those with metastatic sRCC were 23.0, 12.9 and 1.9%, respectively. There were 32 patients with non-metastatic sRCC and their 1-, 2- and 5-year survival rates were 45.5%, 29.9% and 17.9%, respectively.
FIG. 1.
Overall survival for patients with sarcomatoid RCC after nephrectomy (n = 104).
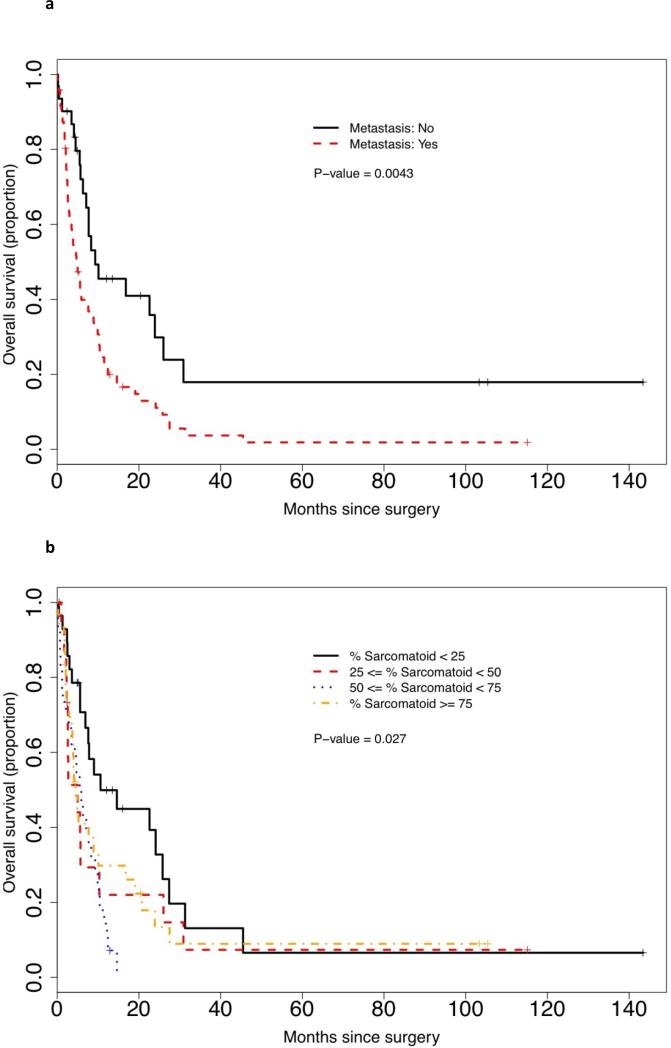
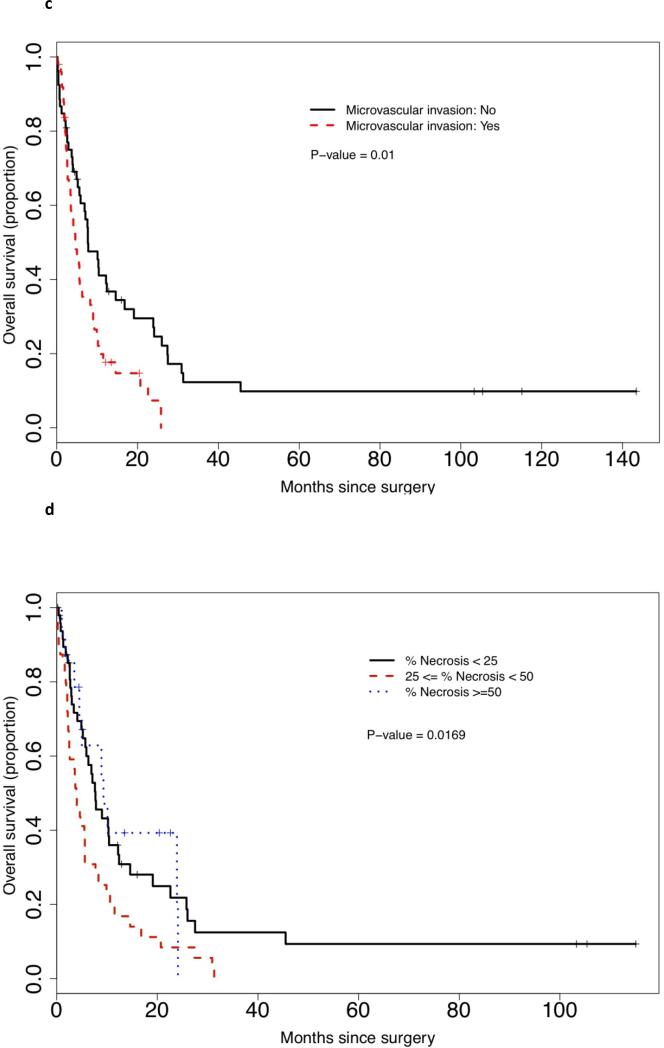
Univariate analysis of survival showed that stage (T1/2, T3 and T4), histology (clear cell vs other), carcinoma grade (2, 3 or 4), and sarcomatoid pattern (defined pattern vs mixed/NOS) did not influence survival (data not shown). ECOGPS (P < 0.001), tumour size (P = 0.021), the presence of MVI (P = 0.01), increased necrosis (by quartile) (P = 0.018), increased PSF (by quartile) (P = 0.027) and metastasic disease (P = 0.004) all had an impact on survival. Kaplan–Meier survival curves for histological features are demonstrated in Figure 2(a)–(d). On multivariate analysis, ECOGPS, tumour size, MVI and metastasis were all retained as independent predictors of poor prognosis, while necrosis approached significance (P = 0.067), and PSF did not reach significance (P = 0.26) (Table 4). A separate univariate and multivariate analysis was performed considering PSF as a continuous variable. Again, while on univariate analysis, increased PSF (continuous) was a predictor of poor outcome (P = 0.045), on multivariate analysis, it approached, but fell short of, statistical significance (P = 0.08).
FIG. 2.
Kaplan-Meier survival curves by (a) the metastatic status; (b) PSF by quartile; (c) the status of MVI; and (d) the percentage necrosis.
TABLE 4.
Multivariate analysis of overall survival
| Value | Hazard ratio | Range | P value |
|---|---|---|---|
| ECOG | |||
| 0 | 1 | ||
| 1 | 2.03 | ||
| 2 | 7.00 | < 0.001 | |
| Tumour size | 1.06 | 1.18–7.26 | 0.045 |
| Microvascular invasion | |||
| No | 1 | ||
| Yes | 1.33 | 0.29 | |
| % necrosis | |||
| < 25% | 1 | ||
| 25 ≤ x < 50% | 1.87 | ||
| ≥ 50% | 1.43 | 0.067 | |
| Percentage sarcomatoid | |||
| < 25% | 1 | ||
| 25 ≤ x < 50% | 1.27 | ||
| 50% ≤ x < 75% | 2.01 | ||
| ≥ 75% | 1.37 | 0.21 | |
| Metastasis | |||
| No | 1 | ||
| Yes | 2.35 | 0.009 |
The extent of the correlation between the multiple clinical variables and survival was demonstrated by the cross-validated ROC analysis, where cross-validated risk score was generated based on the six covariates which were significant in the univariate analysis (ECOGPS, tumour size, necrosis, invasion, PSF-continuous metastasis). The ROC analysis resulted in AUC values of 0.66 (P = 0.03), 0.78 (P < 0.01) and 0.77 (P < 0.01) for survival at 1, 3 and 5 years, respectively.
The patients with non-metastatic sRCC were looked at as a separate cohort for predictors of survival. The PSF (P = 0.011), tumour size (P = 0.015) and ECOGPS (P = 0.028) were significantly associated with outcome on univariate analysis. The multivariate analysis of this small cohort did not identify significant predictors of survival. PSF was not found to be an independent predictor on the multivariate analysis (P = 0.13).
The SEER-17 database analysis found a total of 1005 patients with sRCC (1.6%). Patients with sRCC had very similar characteristics to our cohort with large tumour size (mean 9.9 cm, median 9.0 cm), distant metastasis (54.5%) and the presence of T4 disease (25%). The median cancer-specific survival rates at 1, 2 and 5 years for all patients with sRCC after nephrectomy were 49.8%, 36.6% and 26.8%, respectively. For those with metastatic disease, the 1, 2 and 5-year survival rates were 23.2%, 11.4% and 6%, respectively, while for those with non-metastatic, regional disease, survival rates were 60.6%, 44.6% and 32.0%, respectively.
DISCUSSION
The present study provides a detailed analysis of survival in a large cohort of patients with both metastatic and non-metastatic sRCC. We reinforce the extremely poor outcome of this population, median survival < 6 months for all patients, findings that should reinforce the need for research on the basic biology of sRCC. Unfortunately, limited studies have focused on the biology of sRCC despite being considered to be an extremely aggressive tumour that accounts for up to 20% of patients with stage IV disease [6,13].
In the present study pathological variables were closely analysed to further delineate risk stratification for this patient population. Patients with metastatic disease should be considered to be in the highest risk category as their survival is similar to the poor-risk groups described in other prognostic algorithms [14,15]. For patients with localized disease undergoing nephrectomy, the 1 and 2-year survival rates of 45.5% and 29.9%, respectively, are similar to the survival of patients with non-sRCC undergoing cytoreductive nephrectomy [6]. These patients with non-metastatic sRCC should be informed that surgery likely is the first step of treatment. It is imperative that these patients, especially those with high PSF, be surveyed extremely diligently and strongly encouraged to participate in adjuvant therapy trials.
Several prior series correlated PSF with prognosis and some have demonstrated increased sarcomatoid change to be associated with poor survival [11,16]. To date, no series has identified a defined prognostic threshold, or found this feature to be an independent predictor of survival. In other series, PSF has not been shown to be an important prognostic variable [5,17]. The present data show that while PSF has no association with tumour size, stage, necrosis, MVI or the presence of lymph nodes or metastasis, there was a strong association with survival. Two methods of assessment of PSF (by quartile or as a continuous variable) were performed to determine the influence on outcome. Neither method of characterization could demonstrate that PSF was an independent predictor in our multivariate model; however, as a continuous variable, it approached statistical significance (P = 0.08). For the present cohort of patients with non-metastatic sRCC, PSF was the only significant predictor of survival (P = 0.006) on univariate analysis. The PSF variable was further characterized in the LOOCV ROC analyses and its inclusion was useful at improving the predictive power of our risk score by AUC assessment.
Several studies have shown an interesting biological correlation with a tumour's PSF. Golshayan et al. [7] demonstrated that only patients with a limited amount of sarcomatoid change in the primary tumour (< 20%) had responses with anti-vascular endothelial growth factor therapy. Haas et al. [18] reported that those patients with a high PSF had a greater likelihood of responding to combination chemotherapy with doxorubicin/adriamycin. A possible biological explanation for these findings is related to the increased likelihood of dissemination of the sarcomatoid component in tumours with high PSF, as shown by comparing primary tumours to distant sites of disease.[10]
Another pathological tumour characteristic, MVI, is a strong predictor of survival after nephrectomy in patients with RCC [19,20]. For sRCC, de Peralta-Venturina et al. [11] showed worse survival for those with MVI, but this failed to be an independent predictor of outcome. Cheville et al. [5] did not find MVI to be associated with outcome; however, this series found a very limited number of patients with this pathological characteristic (three of 102; 2.9%). In the present series, there was MVI in 50% of cases and this feature was an independent predictor of poor outcome and useful in our prognostic model. Unfortunately, while recommended by the College of American Pathologists and potentially useful in prognostication, less than half of nephrectomy pathology reports mention this feature.[21]
Many studies with non-sarcomatoid renal tumours have determined that clear-cell histology presents with advanced stage and tumour size and could influence prognosis [22–24]. Recognizable carcinoma elements were identified in over 90% of patients; however, we failed to see any association between histology and clinicopathological variables. Additionally, the present study confirms that histology does not influence survival in patients with sRCC [5,11,16]. As sarcomatoid transformation has been considered to be the final pathway of de-differentiation of renal neoplasms by Delahunt [4], the sarcomatoid biology could dictate outcome and outweigh the originating histology. Despite the lack of influence on survival, reporting the primary histology could still be valuable to treating clinicians and clinical trial design, as those with associated clear-cell histology have had some documented responses to targeted agents [7,25]. The histological pattern of sarcomatoid change did not influence prognosis either and is likely useful only as a descriptive term, especially due to concerns over reproducibility between pathologists.
While there are now a dozen or more prognostic nomograms and algorithms available for RCC after nephrectomy, none is specifically designed for sRCC. A valid question would be why such a model would improve upon the existing clinical tools. Several reasons argue against the existing models in this patient population, including the fact that some of the commonly used models were designed for patients with clear-cell RCC only and have not been tested for those with non-clear-cell sRCC [26,27]. Additionally for such a high-risk population where the median survival is < 6 months, it is unclear how models developed in mostly low- and intermediate-risk patients would perform. Finally, other models of high-risk patients were developed in relation patients with metastatic disease undergoing systemic therapy and cannot be used for those with locally advance/regional tumours [14,15].
The present study has clear limitations due to its retrospective nature and the long study time period (20 years) during which surgical technique, pathological evaluation and systemic treatment strategies have changed. By re-review of all cases by a single genitourinary pathologist, we limit the variability in reporting and update the histology and staging based on current practices. Retrospective tumour assessment does have limitations, as pathological handling (especially renal sinus sampling) has changed over time to stay consistent with existing recommendations. Additionally, retrospective assessment of the PSF is based on all available tumour blocks and ideally would be better estimated if random tumour sampling was performed at the time of specimen procurement.
Another limitation was the use of overall survival rather than cancer-specific survival. While these terms are usually similar for those with aggressive malignancies, we chose overall survival because several patients did not have a documented confirmed cause of death at the time of analysis. Another possible irregularity was that the present cohort had a slightly worse survival than other large series in the literature [5,11,28]. As stage is an important predictor of outcome, it could be due to different patient populations. As the present cohort is derived from a specialized kidney cancer programme, a referral bias could have altered our patient population. However, the data from the present study might be more representative of survival for patients with sRCC, as other series have included patients that were referred after nephrectomy, probably selecting those healthy enough to re-establish care at a new centre [28]. Comparing the present cohort with a nationally representative population listed in SEER, the characteristics and survival appear similar, and therefore we feel this cohort is representative of cases observed in clinical practice. Finally the prognostic model in the present study could prove useful at predicting survival in this population, but it needs external validation and comparison with other clinically relevant survival models. As mentioned earlier, this might be difficult, as many of these prognostic tools are not applicable to those with all histological types and those with localized and metastatic disease.
In conclusion, both patients with localized and metastatic sRCC have very poor survival rates, as demonstrated by the present single institution cohort and cases reported in SEER. While histology and carcinoma grade did not influence survival, other pathological characteristics such as PSF, necrosis and MVI were associated with survival but were not independent predictors. A cross-validated prognostic model for all patients with sRCC was created using ECOGPS, tumour size, MVI, PSF, necrosis and metastatic disease. Patients with localized disease, especially a high PSF, must be either followed extremely closely and/or considered for adjuvant trials.
“What's known on the subject?”.
Sarcomatoid renal cell carcinoma can occur in the setting of all histological subtypes of kidney cancer. These tumours are very aggressive and many patients present with disseminated disease. Long-term survival is poor and the durable responses to systemic therapy are infrequent.
“What does the study add?”.
Our large cohort analyses the influence of pathological tumour characteristics in determining prognosis for patients with sarcomatoid renal cell carcinoma undergoing surgical resection. This series helps define the prognostic influence of histological subtype, type of sarcomatoid morphology, the percentage necrosis and sarcomatoid features, and the presence of microvascular invasion.
Abbreviations
- AUC
area under the curve
- ECOGPS
Eastern Cooperative Group performance status
- LOOCV
leave-one-out cross-validation
- MVI
microvascular invasion
- PSF
percentage of sarcomatoid features
- PSF
percentage of sarcomatoid features
- ROC
receiver operating characteristic
- SEER
Surveillance, Epidemiology, and End Results
- sRCC
sarcomatoid RCC
- sRCC
sarcomatoid renal cell carcinoma
Footnotes
CONFLICT OF INTEREST
None declared. Source of funding: A proportion of research support was provided by the Intramural Research Program of the National Institutes of Health, National Cancer Institute, Center for Cancer Research.
References
- 1.Jemal A, Siegel R, Ward E, Murray T, Xu J, Thun MJ. Cancer statistics, 2007. CA Cancer J Clin. 2007 Jan-Feb;57:43–66. doi: 10.3322/canjclin.57.1.43. [DOI] [PubMed] [Google Scholar]
- 2.Storkel S, Eble JN, Adlakha K, et al. Classification of renal cell carcinoma: Workgroup No. 1. Union Internationale Contre le Cancer (UICC) and the American Joint Committee on Cancer (AJCC). Cancer. 1997 Sep 1;80:987–9. doi: 10.1002/(sici)1097-0142(19970901)80:5<987::aid-cncr24>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 3.Kovacs G, Akhtar M, Beckwith BJ, et al. The Heidelberg classification of renal cell tumours. J Pathol. 1997 Oct;183:131–3. doi: 10.1002/(SICI)1096-9896(199710)183:2<131::AID-PATH931>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 4.Delahunt B. Sarcomatoid renal carcinoma: the final common dedifferentiation pathway of renal epithelial malignancies. Pathology. 1999 Aug;31:185–90. doi: 10.1080/003130299104945. [DOI] [PubMed] [Google Scholar]
- 5.Cheville JC, Lohse CM, Zincke H, et al. Sarcomatoid renal cell carcinoma: an examination of underlying histologic subtype and an analysis of associations with patient outcome. Am J Surg Pathol. 2004 Apr;28:435–41. doi: 10.1097/00000478-200404000-00002. [DOI] [PubMed] [Google Scholar]
- 6.Shuch B, Said J, La Rochelle JC, et al. Cytoreductive nephrectomy for kidney cancer with sarcomatoid histology--is up-front resection indicated and, if not, is it avoidable? J Urol. 2009 Nov;182:2164–71. doi: 10.1016/j.juro.2009.07.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Golshayan AR, George S, Heng DY, et al. Metastatic sarcomatoid renal cell carcinoma treated with vascular endothelial growth factor-targeted therapy. J Clin Oncol. 2009 Jan 10;27:235–41. doi: 10.1200/JCO.2008.18.0000. [DOI] [PubMed] [Google Scholar]
- 8.Staehler M, Haseke N, Roosen A, et al. Sorafenib after Combination Therapy with Gemcitabine plus Doxorubicine in Patients with Sarcomatoid Renal Cell Carcinoma: A Prospective Evaluation. Eur J Med Res. Jul 26;15:287–91. doi: 10.1186/2047-783X-15-7-287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tomic D, Wood LS, Gallager E, Rini BI. Metastatic Sarcomatoid Renal Cell Carcinoma Treated With mTOR-Targeted Therapy.. Kindey Cancer Symposium; Chicago, Il. 2010.2010. [Google Scholar]
- 10.Shuch B, Said J, LaRochelle JC, et al. Histologic evaluation of metastases in renal cell carcinoma with sarcomatoid transformation and its implications for systemic therapy. Cancer. 2010 Feb 1;116:616–24. doi: 10.1002/cncr.24768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.de Peralta-Venturina M, Moch H, Amin M, et al. Sarcomatoid differentiation in renal cell carcinoma: a study of 101 cases. Am J Surg Pathol. 2001 Mar;25:275–84. doi: 10.1097/00000478-200103000-00001. [DOI] [PubMed] [Google Scholar]
- 12.Eble J, Sauter G, Epstein J, Sesterhenn I. Pathology and Genetics, Tumors of the Urinary System and Male Genital Organs. World Health Organization, International Agency for Research on Cancer; Lyon, France: 2004. [Google Scholar]
- 13.Margulis V, Matin SF, Tannir N, et al. Surgical morbidity associated with administration of targeted molecular therapies before cytoreductive nephrectomy or resection of locally recurrent renal cell carcinoma. J Urol. 2008 Jul;180:94–8. doi: 10.1016/j.juro.2008.03.047. [DOI] [PubMed] [Google Scholar]
- 14.Heng DY, Xie W, Regan MM, et al. Prognostic factors for overall survival in patients with metastatic renal cell carcinoma treated with vascular endothelial growth factor-targeted agents: results from a large, multicenter study. J Clin Oncol. 2009 Dec 1;27:5794–9. doi: 10.1200/JCO.2008.21.4809. [DOI] [PubMed] [Google Scholar]
- 15.Motzer RJ, Mazumdar M, Bacik J, Berg W, Amsterdam A, Ferrara J. Survival and prognostic stratification of 670 patients with advanced renal cell carcinoma. J Clin Oncol. 1999 Aug;17:2530–40. doi: 10.1200/JCO.1999.17.8.2530. [DOI] [PubMed] [Google Scholar]
- 16.Ro JY, Ayala AG, Sella A, Samuels ML, Swanson DA. Sarcomatoid renal cell carcinoma: clinicopathologic. A study of 42 cases. Cancer. 1987 Feb 1;59:516–26. doi: 10.1002/1097-0142(19870201)59:3<516::aid-cncr2820590327>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 17.Bertoni F, Ferri C, Benati A, Bacchini P, Corrado F. Sarcomatoid carcinoma of the kidney. J Urol. 1987 Jan;137:25–8. doi: 10.1016/s0022-5347(17)43860-2. [DOI] [PubMed] [Google Scholar]
- 18.Haas N, Manola J, Pins M, et al. ECOG 8802: Phase II trial of doxorubicin (Dox) and gemcitabine (Gem) in metastatic renal cell carcinoma (RCC) with sarcomatoid features.. ASCO: GU Cancer Symposium 2009; 2009; Abstract 285. [Google Scholar]
- 19.Goncalves PD, Srougi M, Dall'lio MF, Leite KR, Ortiz V, Hering F. Low clinical stage renal cell carcinoma: relevance of microvascular tumor invasion as a prognostic variable. J Urol. 2004 Aug;172:470–4. doi: 10.1097/01.ju.0000130582.31467.30. [DOI] [PubMed] [Google Scholar]
- 20.Van Poppel H, Vandendriessche H, Boel K, et al. Microscopic vascular invasion is the most relevant prognosticator after radical nephrectomy for clinically nonmetastatic renal cell carcinoma. J Urol. 1997 Jul;158:45–9. doi: 10.1097/00005392-199707000-00013. [DOI] [PubMed] [Google Scholar]
- 21.Shuch B, Pantuck AJ, Pouliot F, et al. Quality of pathological reporting for renal cell cancer: implications for systemic therapy, prognostication and surveillance. BJU Int. Nov 19; doi: 10.1111/j.1464-410X.2010.09871.x. [DOI] [PubMed] [Google Scholar]
- 22.Amin MB, Amin MB, Tamboli P, et al. Prognostic impact of histologic subtyping of adult renal epithelial neoplasms: an experience of 405 cases. Am J Surg Pathol. 2002 Mar;26:281–91. doi: 10.1097/00000478-200203000-00001. [DOI] [PubMed] [Google Scholar]
- 23.Patard JJ, Leray E, Rioux-Leclercq N, et al. Prognostic value of histologic subtypes in renal cell carcinoma: a multicenter experience. J Clin Oncol. 2005 Apr 20;23:2763–71. doi: 10.1200/JCO.2005.07.055. [DOI] [PubMed] [Google Scholar]
- 24.Cheville JC, Lohse CM, Zincke H, Weaver AL, Blute ML. Comparisons of outcome and prognostic features among histologic subtypes of renal cell carcinoma. Am J Surg Pathol. 2003 May;27:612–24. doi: 10.1097/00000478-200305000-00005. [DOI] [PubMed] [Google Scholar]
- 25.Tickoo SK, Alden D, Olgac S, et al. Immunohistochemical expression of hypoxia inducible factor-1alpha and its downstream molecules in sarcomatoid renal cell carcinoma. J Urol. 2007 Apr;177:1258–63. doi: 10.1016/j.juro.2006.11.100. [DOI] [PubMed] [Google Scholar]
- 26.Sorbellini M, Kattan MW, Snyder ME, et al. A postoperative prognostic nomogram predicting recurrence for patients with conventional clear cell renal cell carcinoma. J Urol. 2005 Jan;173:48–51. doi: 10.1097/01.ju.0000148261.19532.2c. [DOI] [PubMed] [Google Scholar]
- 27.Frank I, Blute ML, Cheville JC, Lohse CM, Weaver AL, Zincke H. An outcome prediction model for patients with clear cell renal cell carcinoma treated with radical nephrectomy based on tumor stage, size, grade and necrosis: the SSIGN score. J Urol. 2002 Dec;168:2395–400. doi: 10.1016/S0022-5347(05)64153-5. [DOI] [PubMed] [Google Scholar]
- 28.Mian BM, Bhadkamkar N, Slaton JW, et al. Prognostic factors and survival of patients with sarcomatoid renal cell carcinoma. J Urol. 2002 Jan;167:65–70. [PubMed] [Google Scholar]