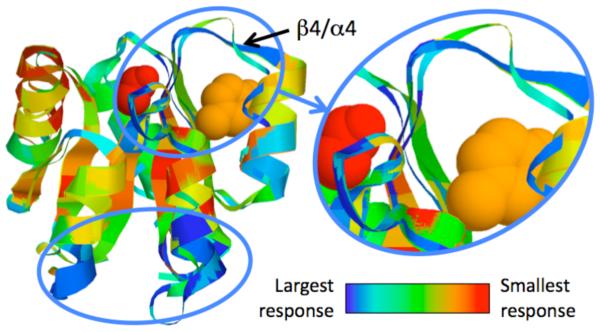
FIGURE 5.
Structure superimposition of 3 CheY orthologs color-coded by changes in cooperativity correlation. Tyr106 (orange) undergoes an allosteric conformational change upon phosphorylation of Asp57 (red) that allows for FliM to bind to CheY, thus relaying the chemotaxis signal. Identified allosteric hotspots are circled, including the β4/α4 loop that is critical to the activation mechanism. Changes in flexibility index are similar, whereas changes in δGfold identify a mostly orthogonal set of allosteric residues.