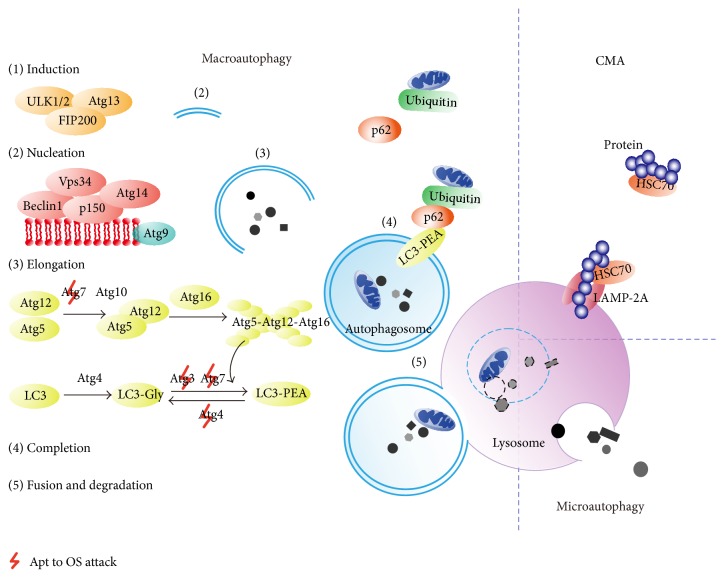
Figure 1.
Mechanisms of autophagy. In macroautophagy, the phagophore is induced by the ULK1/2-Atg13-FIP200 (focal adhesion kinase family-interacting protein of 200 kDa) complex and nucleated by class III phosphatidylinositol 3-kinase (PtdIns3K) complex, which is composed of Vps34 (vacuolar protein sorting 34), Beclin1, p150, and Atg14. Thereafter, Atg4 interacts with LC3 (the microtubule-associated protein light chain 3) to form LC3-I (LC3-Gly) [42, 46, 47]. The Atg12-Atg5-Atg16 complex together with Atg3 and Atg7 stimulates LC3-I to bind with phosphatidylethanolamine (PEA) to produce the LC3-PEA complex (LC3-II), during which the autophagosomal membrane begins to extend and enclose to form an integrated autophagosome [42, 46, 47]. Atg9, like a transport cart, circulates to carry membrane materials for the elongation and expansion of the autophagosomal vesicle [46, 47]. In the end, the mature autophagosome docks and fuses with the lysosome where all of its contents are degraded by acid hydrolases [42, 46, 47]. Macroautophagy can sometimes selectively clear ubiquitinated proteins linked with p62, since p62 works with LC3-II to entrap these long-lived proteins into autophagosomes [48]. In other types of autophagy, such as chaperone-mediated autophagy (CMA), lysosomes selectively degrade cytoplasmic proteins with the KFERQ-related motif, which can be recognized by chaperone HSC70 (heat shock cognate protein 70) [42]. LAMP-2A (lysosome-associated membrane protein 2A) then mediates their entry into lysosomes [42].