Figure 2.
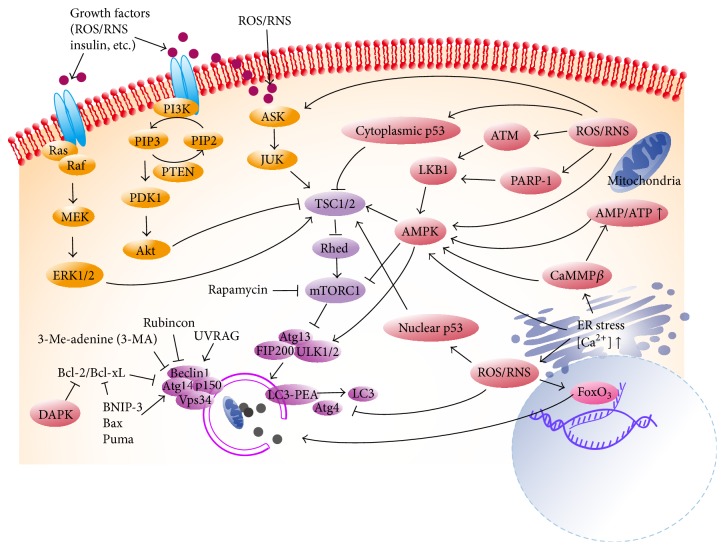
Autophagic signaling pathways associated with oxidative stress. External OS and other stimuli can activate the PI3K-Akt-mTORC1, Ras-MEK-ERK1/2, and ASK- JUK axis [42, 49, 50]. Activated PI3K recruits PDK1 (phosphoinositide-dependent kinase 1) and phosphorylates Akt, inactivating the TSC1/2 (tuberous sclerosis complex 1/2) and leading to mTORC1 activation [42]. mTORC1 is an energy/redox sensor, the function of which can be blocked by rapamycin [42]. It represses autophagy by phosphorylating Atg13 and separating it from the ULK kinase complex [42]. Elevated cytoplasmic ROS/RNS can be sensed by AMPK, ATM kinase, and PARP-1 [47, 51–53]. AMPK activates autophagy by inhibiting mammalian target of rapamycin complex 1 (mTORC1) and directly activating Unc-51-like kinase 1/2 (ULK1/2) [42, 51]. ATM or PARP-1 are able to activate the LKB1 (liver kinase B1)-AMPK-TSC1/2 metabolic pathway to repress mTORC1 or directly activate ULK1/2 through phosphorylation of its serine groups, upregulating autophagy [52, 53]. As well as OS levels, AMPK also responds to cellular AMP/ATP levels, endoplasmic reticulum (ER) stress, and CaMKKβ (calmodulin-dependent protein kinase kinase-β)-related signaling [42, 46, 51]. p53 exhibits paradoxical autophagic regulation in OS, as nuclear p53 positively enhances autophagy through TSC1/2-dependent pathways, whereas cytoplasmic p53 seems to do the opposite [98]. DAPK, BNIP, Bax, and Puma all function to break down the interaction between Bcl-2/Bcl-xL and Beclin-1, normalizing the essential formation of the class III PtdIns3K complex [64, 101, 102]. UVRAG (ultraviolet irradiation resistance-associated gene) also positively regulates this PtdIns3K complex, while Rubicon counteracts its effect [42].