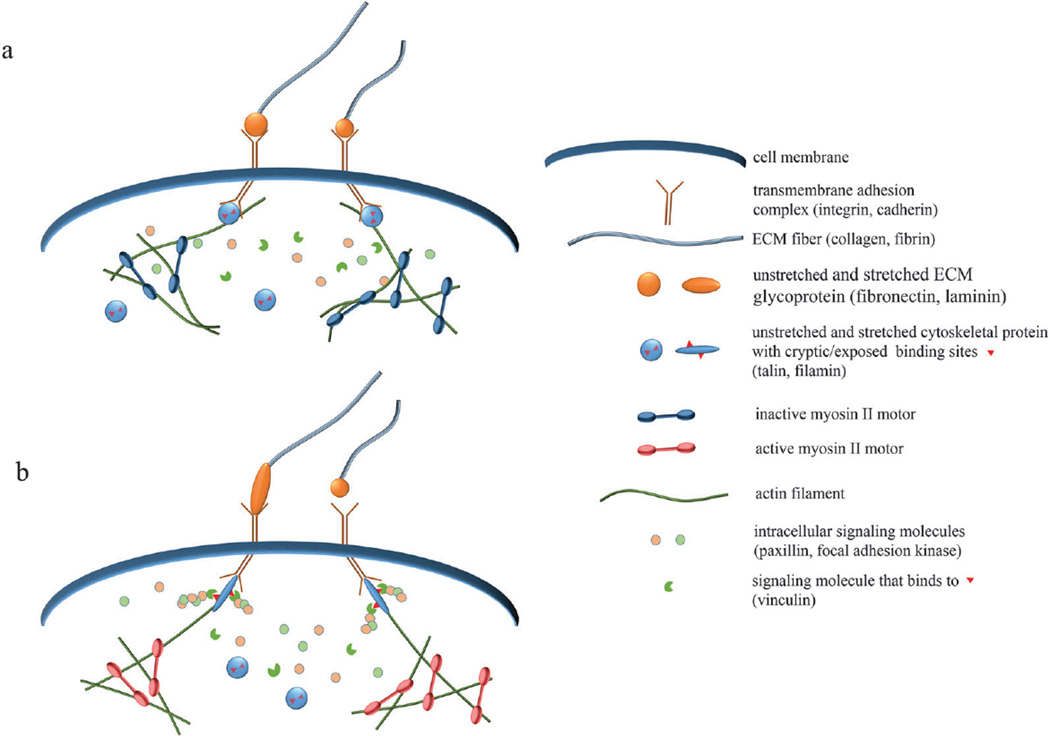
Fig. 2.
Schematics of molecular-level mechanotransduction. (a) When myosin II motors are inactive, cells exhibit reduced tension, causing mechanosensitive protein complexes to assume inactive forms. Inactive talin and filamin have cryptic binding sites, which are unavailable for binding with affiliated molecules. (b) When myosin motors are active or external tension is applied, mechanosensitive proteins become stretched, making accessible previously hidden binding sites. Downstream signaling proteins, such as vinculin, can then bind and activate signaling cascades that promote adhesion, migration, and other physiological functions. Increased force can also alter the binding kinetics and adhesion dynamics of mechanosensitive complexes.