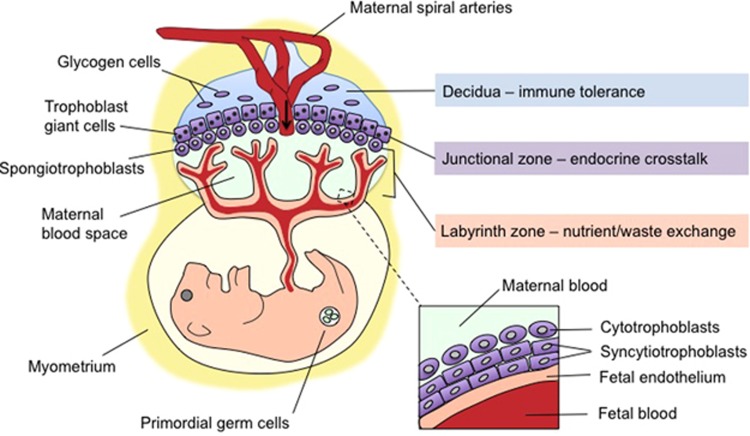
Figure 1.
Morphology of the maternal–fetal interface: navigating a complex interaction between maternal and fetal compartments. Mid-sagittal schematic depicting the intricate arrangement of maternal tissue (blue), fetally derived trophoblasts (all five subtypes in purple), and fetal endothelial cells (tan) within the three major functional zones of the mature mouse placenta. The decidua, the most superficial layer from the maternal side (top), comprises maternal uterine and immune cells, as well as specialized glycogen-storing cells from the trophoblast lineage. The decidua is traversed by the maternal spiral arteries, veins, and the fetally derived endovascular trophoblasts that line it. The arrow indicates the direction of maternal blood flow. Bordering the decidua is the junctional zone (basal plate in humans), where maternal vasculature penetrates a layer of trophoblast giant cells and spongiotrophoblasts (extravillous cytotrophoblasts in humans) that secrete hormones to modulate maternal–fetal cross-talk, angiogenesis, and tissue remodeling. Finally, in the labyrinth zone (chorionic villous in humans), cytotrophoblasts and syncytiotrophoblasts residing between maternal blood spaces and fetal endothelial cells (inset) prevent direct blood contact while facilitating selective and essential nutrient/waste exchange. Environmental stimuli such as maternal stress can disrupt vital aspects of placental organogenesis and function, including decidual immune tolerance, vascularization and utero-placental blood flow, trophoblast hormone secretion, and nutrient exchange within the labyrinth zone. The neurodevelopmental consequences of stress depend on the maturational state of the entire maternal–placental–fetal unit at the time of exposure. Although implantation occurs early in gestation (embryonic day 4.5 in mice and the second week in humans), placental maturation and expansion continues throughout gestation, leaving this fetal lifeline and the somatic and germ cells it sustains continuously vulnerable to maternal stress signals.