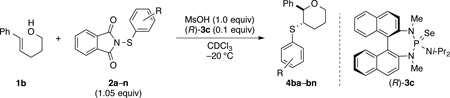
Table 1.
Influence of the sulfenylating agent on enantioselectivity in the sulfenoetherification reaction.
 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| entry | product | conv.a (time) |
e.r.b | entry | product | conv.a (time) |
e.r.b | entry | product | conv.a (time) |
e.r.b |
| 1 | 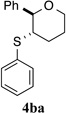 |
93% (24 h) | 95.3:4.7 | 6 | 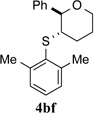 |
100% (21 h) | 97.1:2.9 | 13 | 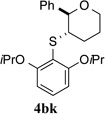 |
80% (24 h) | 98.0:2.0 |
| 2 | 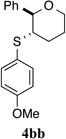 |
100% (24 h) | 95.1:4.9 | 7 | 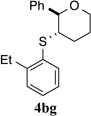 |
>95% (42 h) | 97.5:2.5 | 14 |  |
80% (48 h) | 94.0:6.0 |
| 3 | 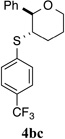 |
>95% (24 h) | 95.6:4.4 | 8 | 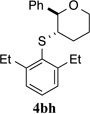 |
>95% (24 h) | 98.0:2.0 | 15 |  |
95% (21 h) | 92.6:7.4 |
| 4 | 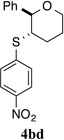 |
88% (51 h) | 95.7:4.3 | 9 |  |
67% (48 h) | 96.3:3.7 | 16 | 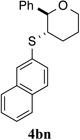 |
90% (48 h) | 91.8:8.2 |
| 5 | 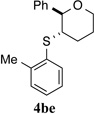 |
>95% (21 h) | 97.2:2.8 | 10 |  |
70% (65 h) | 99.1:0.9 | ||||
| 11 | 80%c (24 h) | 99.0:1.0 | |||||||||
| 12 | 100%d (12 h) | 98.0:2.0 | |||||||||
General reaction conditions: 5 mm NMR tube, 1b (70.0 µmol), 2 (74.0 µmol), MsOH (70.0 µmol), (R)-3c (10 mol%), CDCl3 (0.11 M), −20 °C. Conversion was determined by assuming that, besides small quantities of the respective tetrahydrofuran (<5%), 1b was converted only to 4, as no other significant product was detedcted by 1H NMR spectroscopy.
Determined by CSP-SFC analysis.
Reaction conducted at 0 °C.
Reaction conducted at room temperature.
