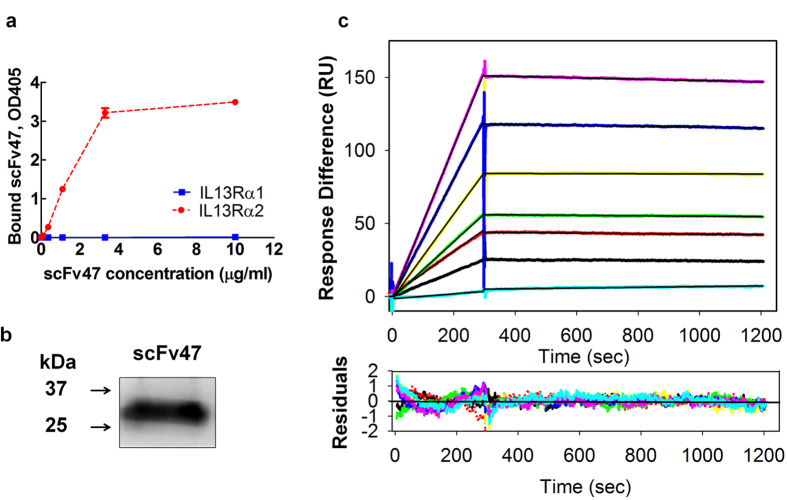
Figure 2. Binding characteristics of scFv47 to IL13Rα2.
(a) Binding of purified soluble scFv47 with rhIL13Rα2 and rhIL13Ra1 proteins was determined in plate ELISA. (b) Western blot analysis of soluble scFv47. The scFv47 protein runs under reducing conditions as a 30kDa protein in agreement with the predicted molecular weight. (c) The kinetics of interactions between the scFv47 and rhIL13Rα2 were visualized by SPR in a Biacore 3000. The scFv47 was injected at concentrations ranging from 1 to 50 nM (lower to upper curves) at a constant flow rate of 20 μL/min over immobilized rhIL13Rα2. The association phase was monitored for 30 sec, dissociation phage for 900 sec following by the change in SPR signal (colored curves), given in RU. Black curves represent the fit of the data to a one-site binding model. For derived kinetic parameters, see Table 1. Lower panels show residuals from the one-site binding model, indicating an excellent fit.