Figure 2.
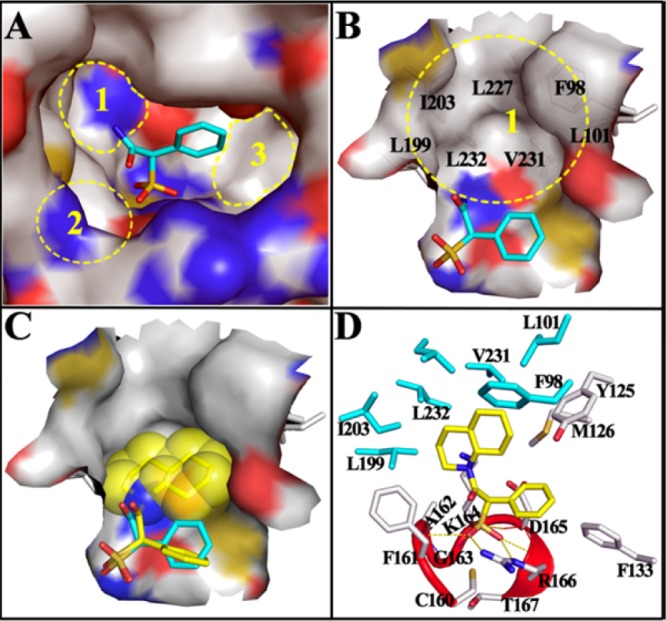
Docking models of SPAA and 9 with mPTPB. (A) SPAA binds into the active site of mPTPB, but there are still multiple free spaces around it. (B) Close-up view of the unoccupied cavity 1, which is mainly constituted by the labeled hydrophobic residues. (C) The binding model of 9 (stick with yellow carbon) with mPTPB. The bound SPAA (stick with cyan carbon) was shown for comparison, and the van der Waals surface of decahydroquinoline was shown in spheres. (D) The interaction details of 9 with mPTPB. The residues within 5 Å distance of 9 are shown in stick, and residues interacting only with 9 but not SPAA are highlighted in cyan. The catalytic P-loop is highlighted in red. H-bonds are indicated by yellow dash lines.
