Abstract
Tetrahydrobiopterin (BH4) is an essential cofactor for the nitric oxide (NO) synthases and the aromatic amino acid hydroxylases. Insufficient BH4 has been implicated in various cardiovascular and neurological disorders. GTP cyclohydrolase 1 (GTPCH-1) is the rate-limiting enzyme for de novo biosynthesis of BH4. The authors have recently shown that the interaction of GTPCH-1 with GTP cyclohydrolase feedback regulatory protein (GFRP) inhibits endothelial GTPCH-1 enzyme activity, BH4 levels, and NO production. They propose that agents that disrupt the GTPCH-1/GFRP interaction can increase cellular GTPCH-1 activity, BH4 levels, and NO production. They developed and optimized a novel time-resolved fluorescence resonance energy transfer (TR-FRET) assay to monitor the interaction of GTPCH-1 and GFRP. This assay is highly sensitive and stable and has a signal-to-background ratio (S/B) greater than 12 and a Z′ factor greater than 0.8. This assay was used in an ultra-high-throughput screening (uHTS) format to screen the Library of Pharmacologically Active Compounds. Using independent protein–protein interaction and cellular activity assays, the authors identified compounds that disrupt GTPCH-1/GFRP binding and increase endothelial cell biopterin levels. Thus, this TR-FRET assay could be applied in future uHTS of additional libraries to search for molecules that increase GTPCH-1 activity and BH4 levels.
Keywords: cardiac diseases, cell-based assays, protein–protein interactions, signal transduction, transcription factors, fluorescence methods
INTRODUCTION
Tetrahydrobiopterin (BH4) is an essential cofactor for all three isoforms of nitric oxide synthases (NOS) and the aromatic amino acid hydroxylases, tryptophan hydroxylase, phenylalanine hydroxylase, and tyrosine hydroxylase. In the case of the NOS enzyme, BH4 is involved in the reduction of a ferrous oxygen complex to an iron-oxy species that hydroxylates L-arginine to produce nitric oxide (NO). In the absence of BH4, the ferrous oxygen complex dissociates to release superoxide, a situation referred to as NOS uncoupling.1
GTP cyclohydrolase 1 (GTPCH-1) converts GTP to dihydroneopterin triphosphate (H2NTP) and is the first and rate-limiting enzyme in the pathway for the de novo synthesis of BH4. The GTP cyclohydrolase 1 feedback regulatory protein (GFRP) is an important modulator of GTPCH-1 enzyme activity. In vitro, GFRP inhibits GTPCH-1 activity in the presence of BH4 by forming a complex with GTPCH-1. This diminishes the Vmax of GTPCH-1, as shown by our own laboratory and others.2,3 Crystal structures of the GTPCH-1/GFRP complex have shown that BH4 is bound at the interface of these two proteins.4 We have recently demonstrated that endogenous GFRP inhibits GTPCH-1 activity, thereby limiting BH4 levels and NO production in human endothelial cells.3 We therefore speculate that measures that cause dissociation of GTPCH-1 and GFRP would increase GTPCH-1 activity and ultimately BH4 production.
Because of its critical role in promoting catalytic activities of NOS and the aromatic amino acid hydroxylases, BH4 deficiency could contribute to a variety of cardiovascular, metabolic, and neurological disorders. As examples, NOS uncoupling due to BH4 deficiency has been documented in experimental models of hypertension,5 atherosclerosis,6 and insulin resistance.7 Unfortunately, oral administration of BH4 is problematic because of its propensity for oxidation, limited bioavailability, and poor cellular uptake. Agents that disrupt the binding of GTPCH-1 and GFRP might therefore have therapeutic potential because they would reduce GFRP inhibition of GTPCH-1 and enhance intracellular BH4 production.
Here, we present details of the development, optimization, and validation of a novel time-resolved fluorescence resonance energy transfer (TR-FRET) assay that monitors the interaction of GTPCH-1 and GFRP. The goal of this TR-FRET assay was to quantitatively measure the GTPCH-1/GFRP interaction and to provide a method that can be used for high-throughput screening (HTS) to identify compounds that disrupt this protein–protein interaction. After optimization, this assay demonstrated an excellent signal-to-background ratio (S/B) and a Z′ factor compatible with an effective HTS. We then used this assay in a 1536-well ultra-HTS (uHTS) format to screen the Library of Pharmacologically Active Compounds (LOPAC). Further validation of positive hits was performed using confirmatory protein–protein interaction and cellular activity assays. This HTS approach could be used to identify small molecules that would be useful in the treatment of diseases associated with BH4 deficiency.
MATERIALS AND METHODS
Basic assay design
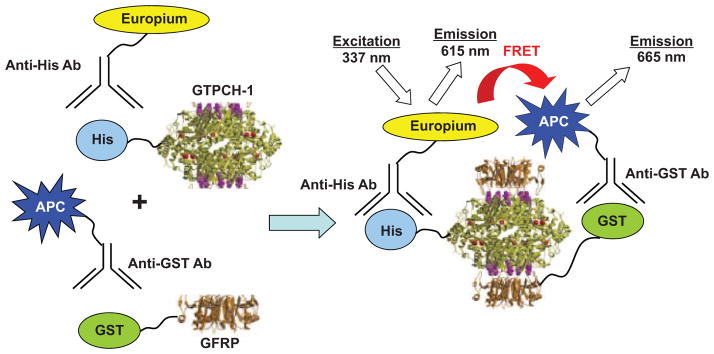
In our TR-FRET assay system, His-tagged human GTPCH-1 is conjugated to a europium chelate via a europium–anti-His antibody as the FRET donor, and glutathione S-transferase (GST)–tagged human GFRP is conjugated to an allophycocyanin (APC) flurophore via an APC–anti-GST antibody as the FRET acceptor (Fig. 1). The binding of GTPCH-1 to GFRP in the presence of necessary cofactors brings europium and APC into close proximity so that energy transfer from an excited-state europium to APC occurs. The europium chelate functions as a long-lifetime (e.g., >100 ms) fluorescent donor, whereas APC is a conventional fluorophore with a short lifetime (e.g., <100 ns). By exciting the europium complex with pulsed light and gating the emission with a 50-μs delay, background emission from the short lifetime acceptor can be eliminated.8 The ratio of the emission of APC at 665 nm and europium at 615 nm reflects the extent of the interaction between GTPCH-1 and GFRP.
FIG. 1.
Design of a time-resolved fluorescence resonance energy transfer (TR-FRET) assay for monitoring the interaction of GTP cyclohydrolase 1 (GTPCH-1) and GTP cyclohydrolase feedback regulatory protein (GFRP). The assay measures the TR-FRET signal upon the binding of a His-tagged GTPCH-1 protein conjugated with a europium–anti-His antibody and glutathione S-transferase (GST)–GFRP conjugated with an allophycocyanin (APC)–anti-GST antibody. Binding of GTPCH-1 and GFRP allows energy transfer from europium to APC and increases the fluorescence emission at 665 nm.
Reagents
Europium–anti-His and allophycocyanin (APC)–anti-GST antibodies were purchased from PerkinElmer (Waltham, MA). Library of Pharmacologically Active Compounds (LOPAC) was from Sigma-Aldrich (St Louis, MO). All other reagents were of highest purity and were obtained from Sigma-Aldrich.
DNA constructs
Human GTPCH-1 cDNA was obtained form GeneCopoeia (cat. no. EX-X0381-M07) and was cloned into the pET-30a(+) vector (EMD Biosciences, Madison, WI) to create the pET-30a/GTPCH-1 construct. The human GFRP cDNA was synthesized by GenScript (Piscataway, NJ) and cloned into the pGEX-5X-3 vector (GE Healthcare, Piscataway, NJ) to create the pGEX-5X-3/GFRP construct. DNA sequencing was used to confirm construct sequences (Agencourt Biosciences, Beverly, MA).
Protein expression and purification of recombinant proteins
Escherichia coli (strain BL21) was transformed with pET-30a/GTPCH-1 and pGEX-5X-3/GFRP constructs and cultured overnight in LB medium. The culture was then diluted 1:100 with prewarmed LB and incubated at 37 °C with vigorous shaking. Recombinant His-GTPCH-1 and GST-GFRP were obtained by isopropyl-β-d-thiogalactopyranoside (IPTG; 0.5 mmol/L) induction for 4 h at 30 °C. His-GTPCH-1 was purified by passing through a nickel column, and GST-GFRP was purified by using the B-PER GST Fusion Protein Purification Kit (Thermo Scientific, Rockford, IL).
TR-FRET measurements
The assay buffer (FRET buffer) contained 20 mM Tris (pH 7.5), 0.01% Nonidet P40, and 50 mM NaCl. Black 384-well and 1536-well microplates (Corning Costar, Cambridge, MA) were used and FRET signals were recorded on an EnVision Multilabel plate reader (PerkinElmer). Europium was excited at 337 nm (laser), and emission from europium donor and APC acceptor was measured at 615/7.5 and 665/7.5 nm, respectively. All FRET signals were expressed as FRET ratios, such that FRET = Emission665 nm/Emission615 nm × 104. The FRET signal window was considered the difference between the maximal FRET value recorded for bound GTPCH-1/GFRP and the minimal FRET value (background), recorded in the absence of GFRP.
Assay development and optimization
The TR-FRET assay was first performed in 384-well microplates with a total volume of 30 μL per well. To determine the binding affinity of GTPCH-1 to GFRP, His-GTPCH-1 was serially diluted into FRET buffer and mixed with GST-GFRP (80 nmol/L), cofactors for GTPCH-1/GFRP interaction (10 μmol/L BH4, 100 μmol/L GTP, and 100 μmol/L ascorbic acid), europium–anti-His antibody (1 nmol/L), and APC–anti-GST antibody (50 nmol/L). A 30-μL volume of this mixture was dispensed into each well, incubated at room temperature for up to 20 h, and measured on the EnVision Multilabel plate reader. Likewise, the binding affinity of GST-GFRP to His-GTPCH-1 was determined by serial dilutions of GST-GFRP into FRET buffer, containing 80 nmol/L His-GTPCH-1, 10 μmol/L BH4, 100 μmol/L GTP, 100 μmol/L ascorbic acid, 1 nmol/L europium–anti-His antibody, and 50 nmol/L APC–anti-GST antibody.
To determine the assay performance for HTS, the signal-to-background ratio and the Z′ factor were calculated based on the following equations: S/B = μb/μf, where μb and μf are the FRET signals for bound (b) GTPCH-1/GFRP and free (f) GTPCH-1 or GFRP alone, respectively. The difference between mean signals from bound and free was represented by (μb-μf). The Z′ factor was calculated using the following equation: Z′ = 1 – (3 × SDb + 3 × SDf)/(μb-μf), where SDb and SDf are the standard deviations for bound (b) and free (f) conditions.9 The Z′ factor reflects the quality of the assay and quantifies the suitability of a particular assay for use in HTS. A Z′ factor between 0.5 and 1.0 indicates an excellent assay for HTS. Assay stability was evaluated by monitoring interaction of GTPCH-1 and GFRP after incubation times from 10 min to 20 h. The effect of DMSO on the maximal signal was evaluated by increasing solvent percentage up to 10% and measuring after a 5-h incubation at room temperature.
HTS format
The suitability of the TR-FRET assay for ultra-HTS (uHTS) was determined by performing the assay in a 1536-well plate format. All reaction volumes were scaled down to a total of 5 μL per well in a 1536-well plate. His-GTPCH-1 (80 nmol/L), GSTGFRP (80 nmol/L), europium–anti-His antibody (1 nmol/L), and APC–anti-GST antibody (50 nmol/L) were diluted in FRET buffer and mixed together. The reaction mixture (5 μL) was dispensed to 1536-well plates, and FRET signals were measured using the EnVision Multilabel plate reader with laser excitation at 337 nm. The S/B ratios and Z′ factors were calculated as described above.
Dose responses were examined to confirm positive compounds from the initial HTS screening and to identify those with potencies in the micromolar range. For the IC50 determinations, serial dilutions of compounds were performed in 100% DMSO with a twofold dilution scheme starting at the highest concentration at 50 μmol/L in the final assay. The assay was performed at 2% DMSO final concentration using conditions described for the primary HTS screen.
To validate the HTS assay and identify active compounds, pilot screening using the LOPAC library was carried out in a 1536-well format. Briefly, the assay mixture was dispensed at 4.5 μL per well into 1536-well black assay plates. The library compounds were added as 0.1 μL of 1-mmol/L DMSO stocks using a pintool integrated with Beckman NX (Beckman Coulter, Brea, CA) to reach a final compound concentration of 21.7 μmol/L. The final DMSO concentration was 2.2% (v/v). To test the reproducibility of the assay, pilot screening was carried out at four replicates per sample. As a control, we included wells containing only one binding partner and DMSO without testing compounds. Assay plates were incubated at room temperature for 2 h, and the FRET signal was measured using the EnVision Multilabel plate reader with a laser excitation at 337 nm and emissions at 615/7.5 nm and 665/7.5 nm.
GST pull-down assay
To validate the identified positive compounds from TR-FERT assay, we developed a GST pull-down assay to test the effect of compound on the dissociation of GFRP and GTPCH-1 binding in vitro. To detect association of GST-GFRP with His-GTPCH-1, the ProFound Pull-Down GST Protein:Protein Interaction Kit (Thermo Scientific) was employed. Purified GST-GFRP recombinant protein (0.7 μg) was first incubated with glutathione agarose beads for 1 h at 4 °C and then incubated with 0.7 μg of purified His-GTPCH-1 recombinant protein in the FRET buffer containing 10 μmol/L BH4, 100 μmol/L GTP, and 100 μmol/L ascorbic acid. Protein complexes were eluted from glutathione and then separated on a 12.5% gel by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PA GE), followed by Western blot analysis using anti-His tag and anti-GFRP antibodies.
Cell culture and cellular BH4 measurement
Human aortic endothelial cells (HAECs) were purchased from Lonza (Walkersville, MD) and used between the third and sixth passages. Cellular biopterins (BH4 and more oxidized species) were measured by high-performance liquid chromatography (HPLC) as previously described.10
Data analysis
Assay data were analyzed using Bioassay software from CambridgeSoft (Cambridge, MA). The effect of compounds on the binding of GFRP and GTPCH-1 was expressed as % of Control and was calculated with the following equations based on data from each plate: % of Control = (FRETcompound – FRETbackground)/(FRETcontrol – FRETbackground) × 100, where FRETcompound is the FRET ratio from a well with a test compound. FRETcontrol is an average FRET signal from wells without a test compound, which defines maximal FRET. FRETbackground is an average FRET ratio from wells lacking GST-GFRP, which defines minimal FRET. Compounds that caused % Control <70 were defined as positives. IC50 values were defined as the concentration of compounds that caused a 50% reduction in assay signal.
Data were expressed as mean ± standard error of the mean. Analysis of variance (ANOVA) was employed for multiple comparisons, and when one group was served as a control, the Dunnett post hoc test was used. A value of p < 0.05 was considered significant.
RESULTS
Development of the TR-FRET GTPCH-1/GFRP binding assay
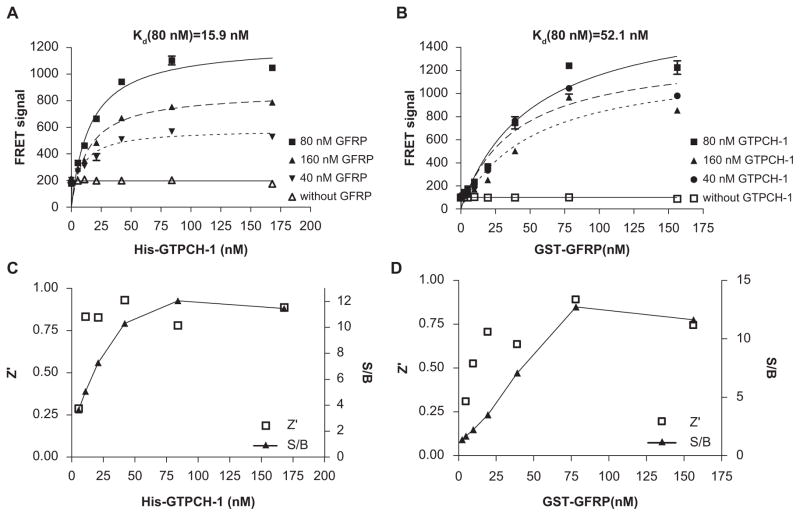
To develop a TR-FRET assay that monitors the interaction of GTPCH-1 and GFRP, titrations of GTPCH-1 and GFRP proteins were carried out to determine the conditions that generate an optimal TR-FRET signal. As shown in Figure 2A, B, increasing concentrations of either His-GTPCH-1 or GST-GFRP dose-dependently increased the TR-FRET signal. The TR-FRET signal reached a maximum at approximately 80 nmol/L for both proteins. The fact that equal molar amounts of GTPCH-1 and GFRP were required to produce a maximal FRET value is in accordance with the previous finding that the ratio of GTPCH-1 and GFRP in the GTPCH-1/GFRP complex is 1:1.11 In keeping with this, the Kd values for binding of GTPCH-1 with GFRP and for GFRP with GTPCH-1 were similar (15.9 and 52.1 nmol/L, respectively; Fig. 2A,B). In both titrations, the quality of the assay was evaluated by calculating S/B ratios and Z′ factors over the concentration ranges. A maximal S/B ratio of 12 was obtained at 80 nM of His-GTPCH-1 and GST-GFRP, and the Z′ factor was approximately 0.8 at these concentrations (Fig. 2C,D). The Z′ factor of an assay reflects the variability of an assay and the suitability for HTS, and a Z′ factor between 0.5 and 1 reflects a reliable and robust assay.9 The Z′ factor of 0.8 for our assay indicates that it is very effective.
FIG. 2.
Optimization of the GTP cyclohydrolase 1 (GTPCH-1)/GTP cyclohydrolase feedback regulatory protein (GFRP) time-resolved fluorescence resonance energy transfer (TR-FRET) binding assay. (A) Effect of titrations of His-GTPCH-1 into solutions of glutathione S-transferase (GST)–GFRP, europium–anti-His, and allophycocyanin (APC)–anti-GST with the necessary cofactors for binding. (B) Effect of titrations of GST-GFRP into solutions of His-GTPCH-1, europium–anti-His, and APC–anti-GST with the necessary cofactors for binding. (C) Z′ factors and signal-to-background (S/B) ratios across the His-GTPCH-1 titration. (D) Z′ factors and S/B ratios across the GST-GFRP titration.
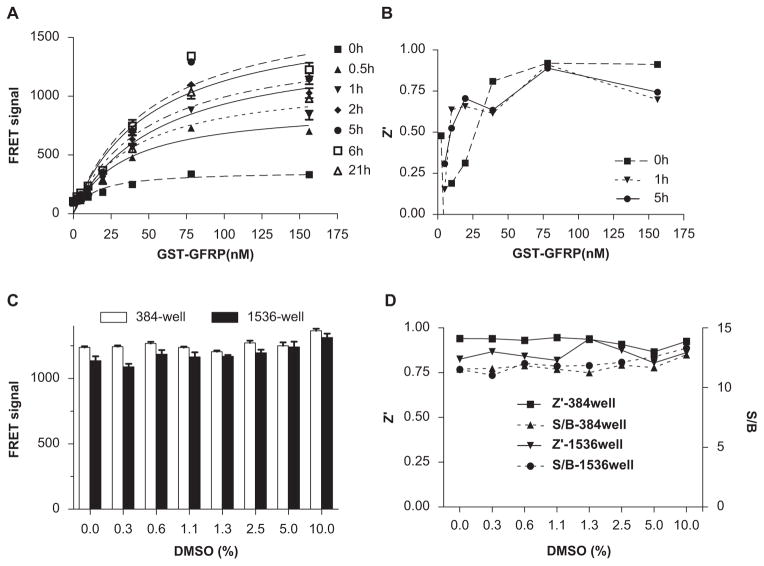
The FRET signal increased progressively up to 6 h after mixing GTPCH-1, GFRP, and cofactors and decreased slightly thereafter (Fig. 3A). Thus, this assay provides sufficient stability for compound screening. HTS often employs compound libraries that are dissolved in DMSO. We therefore tested assay tolerance to DMSO at concentrations commonly used in screening. There was no appreciable decline in FRET signal with up to 10% DMSO in the 384-well plate format (Fig. 3B). The S/B ratios and Z′ factors remained constant at various DMSO concentrations (Fig. 3C). The use of high-density microplates, most prominently 1536-well plates, allows miniaturizing screening volumes to realize proportional savings in reagent costs. We therefore examined the assay performance in a 1536-well plate format. There were no appreciable changes in assay signal, S/B ratio, or Z′ factor in response to increasing concentrations of DMSO in the 1536-well plate format (Fig. 3B,C). This suggests that after optimization, the TR-FRET assay to monitor the GTPCH-1/GFRP interaction is suitable for uHTS in a 1536-well plate format. Taken together, these results demonstrate that this TR-FRET assay provides a simple, reliable, and robust readout for monitoring the GTPCH-1/GFRP interaction and discovering small molecules that disrupt this interaction.
FIG. 3.
Time-resolved fluorescence resonance energy transfer (TR-FRET) assay stability. (A) TR-FRET signals of glutathione S-transferase (GST)–GTP cyclohydrolase feedback regulatory protein (GFRP) titration assay at various time points. (B) Z′ factors across the time course of the assay. (C) Effect of various concentrations of DMSO on the FRET signal using the 384-well and 1536-well plate formats. (D) Z′ factors and signal-to-background (S/B) ratios for assay with increasing DMSO concentrations using the 384-well and 1536-well plate formats.
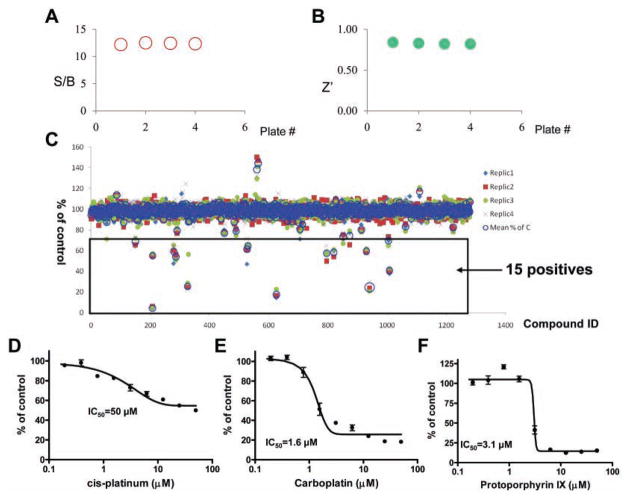
Assay validation of uHTS for GTPCH-1/GFRP interaction inhibitors: pilot screening of the LOPAC library
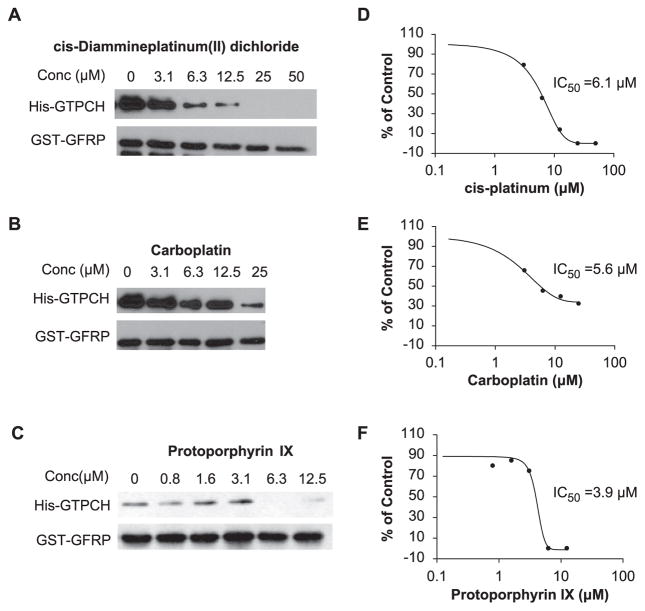
The GTPCH-1/GFRP TR-FRET assay was validated for use in an uHTS format by a pilot screening of LOPAC, which is a collection of 1280 pharmacologically active compounds frequently used for HTS assay validation.12,13 The average S/B ratio and Z′ factor observed during LOPAC screening were consistent with our assay validation studies (Fig. 4A,B). Compounds that inhibited the binding of GTPCH-1 to GFRP by more than 30% were considered potential positives, and the screening results for the LOPAC library are shown in Figure 4C. Of the 1280 compounds, 15 were identified as potential positives, which resulted in a hit rate of 1.2%. Subsequent dose-response curves for these positive compounds were performed, and representative results for three positives are shown in Figure 4D–F. These compounds—cis-diammineplatinum(II) dichloride, carboplatin, and protoporphyrin IX—were confirmed to inhibit the interaction of GTPCH-1 and GFRP with IC50s in the micromolar range. These findings suggest that this TR-FRET assay, developed to discover compounds that inhibit the binding of GTPCH-1 and GFRP, is suitable for HTS and can be adapted for large-scale compound screening.
FIG. 4.
Ultra-high-throughput screening (uHTS) format assay validation. The time-resolved fluorescence resonance energy transfer (TR-FRET) assay that monitors GTP cyclohydrolase 1 (GTPCH-1)/GTP cyclohydrolase feedback regulatory protein (GFRP) interaction was validated for uHTS using the Library of Pharmacologically Active Compounds (LOPAC) library in four replicates. Signal-to-background (S/B) ratios (A) and Z′ factors (B) were evaluated for four plates. (C) The percentage of control is calculated as defined in the Materials and Methods section and plotted against compound ID. The potential positives are defined by the compound with % inhibition >30. Representative dose-response curves of the potential positive compounds determined by the TR-FRET assay are shown in panels D, E, and F.
Conformation of the hits from the LOPAC pilot screening
We further examined the ability of cis-diammineplatinum(II) dichloride, carboplatin, and protoporphyrin IX to disrupt the interaction of GTPCH-1 and GFRP in a GST pull-down assay (Fig. 5A–C). Dose-response curves and IC50 values of the compounds were obtained from the densitometry of the GST pull-down assay (Fig. 5D–F). The GST pull-down assay confirmed that these compounds can inhibit the interaction of GTPCH-1 and GFRP dose-dependently in the low micromolar range.
FIG. 5.
Validation of positive compounds in the glutathione S-transferase (GST) pull-down assay. GST–GTP cyclohydrolase feedback regulatory protein (GFRP) was bound to glutathione agarose beads, and then His-tagged GTP cyclohydrolase 1 (GTPCH-1) was added. These experiments were performed in the presence of various concentrations of compounds identified by initial high-throughput screening (HTS) of the Library of Pharmacologically Active Compounds (LOPAC) library. The Western blot in the lower panel shows the amount of GFRP used as “bait,” whereas the “trapped” GTPCH-1 is shown in the upper blot. The disappearance of a band in the upper panel indicates disruption of GTPCH/GFRP binding. Panels A, B, and C show the representative GST pull-down assay testing the potential positive compounds. Panels D, E, and F show the representative dose-response curves determined from the densitometry of the GST pull-down assays.
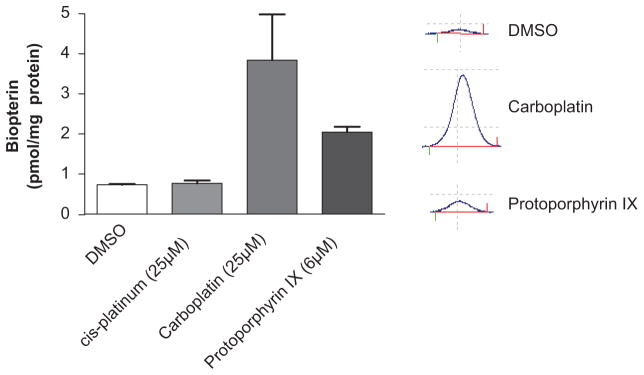
Given these results, we then performed additional studies to evaluate the ability of these compounds to increase endothelial levels of BH4. The above identified agents were added to cultured human aortic endothelial cells for 24 h, and subsequent changes in biopterin levels were measured using HPLC. We found that carboplatin and protoporphyrin IX increased biopterin levels in cultured endothelial cells at low micromoloar concentrations, with carboplatin eliciting a fourfold increase in biopterin levels at the concentration of 25 μmol/L (Fig. 6). These results demonstrate the feasibility of the TR-FRET assay and subsequent confirmatory steps to identify compounds that can enhance endogenous production of BH4.
FIG. 6.
Effect of the primary hits on endothelial cell biopterin levels. Human aortic endothelial cells were treated with compounds at the concentrations indicated for 24 h, and biopterin levels were measured using high-performance liquid chromatography (n = 6). Insets on the right show representative biopterin peaks for cells treated with DMSO, carboplatin, or protoporhyrin XI at indicated concentrations. Values were compared using analysis of variance, and selected comparisons were made using a Dunnett post hoc test.
DISCUSSION
In the present study, we have designed, optimized, and validated a homogeneous TR-FRET assay to monitor the interaction of GTPCH-1 and GFRP proteins. The goals in establishing a robust and simple to use assay for measuring GTPCH-1 and GFRP interactions were, first, to allow the quantification of this interaction in vitro and, second, to optimize conditions for identifying small-molecule regulators of this interaction in an HTS format. This TR-FRET assay demonstrates a good Z′ factor and S/B ratio and exhibits high stability, solvent tolerance, and good assay performance in small volumes, indicating that it is particularly well suited for uHTS in a 1536-well format. This assay can be performed in a simple “mix-and-read” approach and has been validated as robust and reliable for discovery of small molecules that disrupt the GTPCH-1/GFRP interaction. We further confirmed the utility of this assay by studying identified compounds in a GST pull-down assay and in cell culture. Thus, our newly developed TR-FRET assay might prove useful in screening larger chemical libraries to identify compounds that could increase cellular BH4 levels. To our knowledge, this is the first TR-FRET assay developed for monitoring the interaction of GTPCH-1 and GFRP and for discovery of small molecules that disrupt this interaction.
As a second approach for compound screening, we employed a GST pull-down assay and identified three compounds that dose-dependently inhibited the GTPCH-1/GFRP interaction in vitro. The false positives from HTS might be caused by their interference with fluorescence in the TR-FRET assay, and this can be eliminated by testing the compounds using the GST pull-down assay. Some compounds might also interfere with antibody binding to the target proteins and thus would artificially disrupt the TR-FRET signal. Such compounds would also be identified as false positives. These considerations emphasize the need for the secondary screening using an independent assay such as the GST pull-down approach. Cellular uptake of compounds might also be limited, and therefore further screening using cultured cells is necessary.
Using these sequential screening approaches, we identified two compounds that can increase endothelial BH4 levels at low micromolar concentrations. Carboplatin is an alkylating agent used for a variety of cancers, including ovarian and lung carcinoma.4 It has significant side effects, including severe myelosuppression. Interestingly, higher concentrations of carboplatin failed to elicit an increase in cellular biopterin levels. This is possibly due to the cellular toxicity of this agent at higher concentrations. It is conceivable that modification of the carboplatin structure could yield compounds that would increase cellular BH4 without toxicity. Protoporphyrin IX is a precursor to heme, myoglobin, and other compounds such as catalase. Protoporphyrin IX has been used as a specific probe to activate heme oxygenase. 14 Our studies indicate that it might have additional effects in increasing cellular BH4 and activities of BH4-dependent enzymes, such as NOS. We have not examined the effectiveness of these specific drugs in vivo and therefore do not propose that they have therapeutic benefit in increasing cellular BH4 in intact animals. Our proof-of-principle study does show that this optimized uHTS approach can be used to screen larger libraries for discovery of other molecules that increase endogenous production of BH4.
The mechanisms by which these compounds dissociate GTPCH-1 and GFRP remain unknown. A common mechanism for inhibiting protein–protein interaction is that small molecules bind at the interface of the interaction. Crystal structure analysis of GTPCH-1/GFRP complex suggests that that Arg235 and Glu236 of GTPCH-1 in the C-terminal region directly interact with GFRP.11 A yeast two-hybrid assay15 and studies from our own laboratory (data not shown) have demonstrated that the complete N-terminal peptide of GTPCH-1 is required for interaction with GFRP. Compounds that interfere with binding between these regions could disrupt the GTPCH-1/GFRP interaction. In addition, the compounds could also cause allosteric inhibition by inducing conformational changes of the proteins. Further investigation using methods that address the compound binding to the proteins, such as X-ray crystallography, would help define the exact mechanisms of the action of these compounds.16 These identified compounds might serve as tools to facilitate the further study of the GPTCH-1 and GFRP interaction.
BH4 is an essential cofactor for NOS and a key regulator of NOS activity and coupling. BH4 also functions as a critical cofactor for the aromatic amino acid hydroxylases and thus modulates several neurological processes. BH4 deficiency has been found in experimental models of various cardiovascular diseases, such as hypertension,5 atherosclerosis,17 and diabetes.7 Oral BH4 supplementation has been shown effective in ameliorating these conditions in animal models,5,18 but the effect of BH4 in humans is likely limited because of its poor bioavailability and cellular uptake.19 Accordingly, Antoniades et al.20 reported that patients with coronary artery disease have paradoxically high biopterin levels in the plasma but low biopterin levels in vessels. Thus, compounds identified by our screening approaches to increase intracellular BH4 biosynthesis could have therapeutic potential in treating diseases associated with intracellular BH4 deficiency.
In summary, we have successfully developed a one-step mix-and-read TR-FRET assay for monitoring the interaction of GTPCH-1 and GFRP. This TR-FERT assay is highly sensitive and stable and exhibits robust performance in an uHTS format with an S/B ratio greater than 12 and a Z′ factor greater than 0.8. We have employed this assay in uHTS, and results from a pilot screening showed that positive hits from the primary screening exhibit activity in an independent protein binding assay and in cell culture. Screening of larger chemical libraries and additional structure activity work are needed to identify compounds that could be used therapeutically.
Acknowledgments
This work was supported by National Institutes of Health (NIH) grants P01HL58000 and R01HL39006 (to D.G.H.) and an American Heart Association Predoctoral Grant (to L.L.), Emory URC (to Y.D.), Georgia Cancer Coalition, Georgia Research Alliance, and Emory Faculty Distinction Fund (to H.F.).
References
- 1.Vasquez-Vivar J, Kalyanaraman B, Martasek P. The Role of Tetrahydrobiopterin in Superoxide Generation from eNOS: Enzymology and Physiological Implications. Free Radic Res. 2003;37:121–127. doi: 10.1080/1071576021000040655. [DOI] [PubMed] [Google Scholar]
- 2.Harada T, Kagamiyama H, Hatakeyama K. Feedback Regulation Mechanisms for the Control of GTP Cyclohydrolase I Activity. Science. 1993;260:1507–1510. doi: 10.1126/science.8502995. [DOI] [PubMed] [Google Scholar]
- 3.Li L, Rezvan A, Salerno JC, Husain A, Kwon K, Jo H, Harrison DG, Chen W. GTP Cyclohydrolase I Phosphorylation and Interaction with GTP Cyclohydrolase Feedback Regulatory Protein Provide Novel Regulation of Endothelial Tetrahydrobiopterin and Nitric Oxide. Circ Res. 2010;106:328–336. doi: 10.1161/CIRCRESAHA.109.210658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yoneyama T, Hatakeyama K. Decameric GTP Cyclohydrolase I Forms Complexes with Two Pentameric GTP Cyclohydrolase I Feedback Regulatory Proteins in the Presence of Phenylalanine or of a Combination of Tetrahydrobiopterin and GTP. J Biol Chem. 1998;273:20102–20108. doi: 10.1074/jbc.273.32.20102. [DOI] [PubMed] [Google Scholar]
- 5.Landmesser U, Dikalov S, Price SR, McCann L, Fukai T, Holland SM, Mitch WE, Harrison DG. Oxidation of Tetrahydrobiopterin Leads to Uncoupling of Endothelial Cell Nitric Oxide Synthase in Hypertension. J Clin Invest. 2003;111:1201–1209. doi: 10.1172/JCI14172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Channon KM. Tetrahydrobiopterin: Regulator of Endothelial Nitric Oxide Synthase in Vascular Disease. Trends Cardiovasc Med. 2004;14:323–327. doi: 10.1016/j.tcm.2004.10.003. [DOI] [PubMed] [Google Scholar]
- 7.Du X, Edelstein D, Obici S, Higham N, Zou MH, Brownlee M. Insulin Resistance Reduces Arterial Prostacyclin Synthase and eNOS Activities by Increasing Endothelial Fatty Acid Oxidation. J Clin Invest. 2006;116:1071–1080. doi: 10.1172/JCI23354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gunther JR, Du Y, Rhoden E, Lewis I, Revennaugh B, Moore TW, Kim SH, Dingledine R, Fu H, Katzenellenbogen JA. A Set of Time-Resolved Fluorescence Resonance Energy Transfer Assays for the Discovery of Inhibitors of Estrogen Receptor-Coactivator Binding. J Biomol Screen. 2009;14:181–193. doi: 10.1177/1087057108329349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ozers MS, Ervin KM, Steffen CL, Fronczak JA, Lebakken CS, Carnahan KA, Lowery RG, Burke TJ. Analysis of Ligand-Dependent Recruitment of Coactivator Peptides to Estrogen Receptor Using Fluorescence Polarization. Mol Endocrinol. 2005;19:25–34. doi: 10.1210/me.2004-0256. [DOI] [PubMed] [Google Scholar]
- 10.Widder JD, Chen W, Li L, Dikalov S, Thony B, Hatakeyama K, Harrison DG. Regulation of Tetrahydrobiopterin Biosynthesis by Shear Stress. Circ Res. 2007;101:830–838. doi: 10.1161/CIRCRESAHA.107.153809. [DOI] [PubMed] [Google Scholar]
- 11.Maita N, Hatakeyama K, Okada K, Hakoshima T. Structural Basis of Biopterin-Induced Inhibition of GTP Cyclohydrolase I by GFRP, Its Feedback Regulatory Protein. J Biol Chem. 2004;279:51534–51540. doi: 10.1074/jbc.M409440200. [DOI] [PubMed] [Google Scholar]
- 12.Benjamin ER, Pruthi F, Olanrewaju S, Ilyin VI, Crumley G, Kutlina E, Valenzano KJ, Woodward RM. State-Dependent Compound Inhibition of Nav1.2 Sodium Channels Using the FLIPR Vm Dye: On-Target and Off-Target Effects of Diverse Pharmacological Agents. J Biomol Screen. 2006;11:29–39. doi: 10.1177/1087057105280918. [DOI] [PubMed] [Google Scholar]
- 13.Rickardson L, Wickstrom M, Larsson R, Lovborg H. Image-Based Screening for the Identification of Novel Proteasome Inhibitors. J Biomol Screen. 2007;12:203–210. doi: 10.1177/1087057106297115. [DOI] [PubMed] [Google Scholar]
- 14.Li C, Stocker R. Heme Oxygenase and Iron: From Bacteria to Humans. Redox Rep. 2009;14:95–101. doi: 10.1179/135100009X392584. [DOI] [PubMed] [Google Scholar]
- 15.Swick L, Kapatos G. A Yeast 2-Hybrid Analysis of Human GTP Cyclohydrolase I Protein Interactions. J Neurochem. 2006;97:1447–1455. doi: 10.1111/j.1471-4159.2006.03836.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Arkin MR, Randal M, DeLano WL, Hyde J, Luong TN, Oslob JD, Raphael DR, Taylor L, Wang J, McDowell RS, et al. Binding of Small Molecules to an Adaptive Protein-Protein Interface. Proc Natl Acad Sci U S A. 2003;100:1603–1608. doi: 10.1073/pnas.252756299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Takaya T, Hirata K, Yamashita T, Shinohara M, Sasaki N, Inoue N, Yada T, Goto M, Fukatsu A, Hayashi T, et al. A Specific Role for eNOS-Derived Reactive Oxygen Species in Atherosclerosis Progression. Arterioscler Thromb Vasc Biol. 2007;27:1632–1637. doi: 10.1161/ATVBAHA.107.142182. [DOI] [PubMed] [Google Scholar]
- 18.Hattori Y, Hattori S, Wang X, Satoh H, Nakanishi N, Kasai K. Oral Administration of Tetrahydrobiopterin Slows the Progression of Atherosclerosis in Apolipoprotein E-Knockout Mice. Arterioscler Thromb Vasc Biol. 2007;27:865–870. doi: 10.1161/01.ATV.0000258946.55438.0e. [DOI] [PubMed] [Google Scholar]
- 19.Katusic ZS, d’Uscio LV, Nath KA. Vascular Protection by Tetrahydrobiopterin: Progress and Therapeutic Prospects. Trends Pharmacol Sci. 2009;30:48–54. doi: 10.1016/j.tips.2008.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Antoniades C, Shirodaria C, Crabtree M, Rinze R, Alp N, Cunnington C, Diesch J, Tousoulis D, Stefanadis C, Leeson P, et al. Altered Plasma versus Vascular Biopterins in Human Atherosclerosis Reveal Relationships between Endothelial Nitric Oxide Synthase Coupling, Endothelial Function, and Inflammation. Circulation. 2007;116:2851–2859. doi: 10.1161/CIRCULATIONAHA.107.704155. [DOI] [PubMed] [Google Scholar]