Abstract
This study investigated the effects of bone morphogenetic protein 6 (BMP6) supplementation in the medium during in vitro maturation (IVM) on the developmental potential of oocytes and in the subsequent development of cloned yak embryos. Cumulus–oocyte complexes (COCs) were aspirated from the antral follicles of yak ovaries and cultured with different concentrations of recombinant human BMP6 in oocyte maturation medium. Following maturation, the metaphase II (MII) oocytes were used for somatic cell nuclear transfer (SCNT), and these were cultured in vitro. The development of blastocysts and cell numbers were detected on day 8. The apoptosis and histone modifications of yak cloned blastocysts were evaluated by detecting the expression of relevant genes and proteins (Bax, Bcl-2, H3K9ac, H3K18ac, and H3K9me3) using relative quantitative RT-PCR or immunofluorescence. The presence of 100 ng/mL BMP6 significantly enhanced the oocyte maturation ratios (66.12 ± 2.04% vs. 73.11 ± 1.38%), cleavage rates (69.40 ± 1.03% vs. 78.16 ± 0.93%), and blastocyst formation rates (20.63 ± 1.32% vs. 28.16 ± 1.67%) of cloned yak embryos. The total blastocysts (85.24 ± 3.12 vs. 103.36 ± 5.28), inner cell mass (ICM) cell numbers (19.59 ± 2.17 vs. 32.20 ± 2.61), and ratio of ICM to trophectoderm (TE) (22.93 ± 1.43% vs. 31.21 ± 1.62%) were also enhanced (p < 0.05). The ratio of the Bax to the Bcl-2 gene was lowest in the SCNT + BMP6 groups (p < 0.05). The H3K9ac and H3K18ac levels were increased in SCNT + BMP6 groups (p < 0.05), whereas the H3K9me3 level was decreased; the differences in blastocysts were not significant (p > 0.05). These study results demonstrate that addition of oocyte maturation medium with recombinant BMP6 enhances yak oocyte developmental potential and the subsequent developmental competence of SCNT embryos, and provides evidence that BMP6 is an important determinant of mammalian oocyte developmental reprogramming.
Introduction
Enucleated metaphase II (MII) stage oocytes are capable of reprogramming nuclei of differentiated somatic cells into totipotency during somatic cell nuclear transfer (SCNT), thus allowing the cloning of numerous mammalian species (Baguisi et al., 1999; Kato et al., 1998; Lee et al., 2005; Li et al., 2006; Onishi et al., 2000; Wakayama et al., 1998; Wilmut et al., 1999). However, this reprogramming method remains inefficient, and its low efficiency has limited its widespread use (Yang et al., 2007). Oocyte quality is one of the limiting factors that affect the efficiency of animal cloning (Su et al., 2014a, b). The cumulus–oocyte complexes (COCs) of yak (Bos grunniens) are usually collected from yak ovaries at an abattoir and should undergo in vitro maturation (IVM) prior to using in vitro fertilization (IVF) or SCNT. However, the developmental potential of mammalian oocytes matured in vitro is lower compared to oocytes matured in vivo, perhaps due to inadequate support of complete oocyte maturation by the in vitro environments, such as decreased concentration of oocyte-secreted factors (OSFs) (Gilchrist et al., 2008).
Oocytes and their surrounding cumulus cells (CCs) maintain a close association during the period of maturation, which is necessary for both the oocytes as well as CCs development (Buccione et al., 1990). The CCs provide the nutrients and the regulatory signals upon which the oocytes are dependent, and these elements play very crucial roles in the maturation of oocytes cytoplasm and nucleus; in this way, developmental competence is acquired. (Chian and Sirard, 1995; Gilchrist et al., 2008). It has been shown that when CCs are removed at early stage of IVM from oocytes or before the IVF, the rates of maturation, cleavage, and blastocyst formation are reduced. In turn, OSFs are responsible for the granulosa cell (GC) differentiation and development, and they perform various roles in the GCs (Fatehi et al., 2002; Zhang et al., 1995). The effects of OSFs on their surrounding CCs have been widely verified; for example, they promote cell development and prevent cell apoptosis and death (Gilchrist et al., 2004). This communication is important for the maturation of oocytes and subsequent embryogenesis. The studies of Yeo et al. found that the developmental potential of oocytes matured in vitro was reduced by the low levels of OSFs; communication between the oocyte and cumulus was also blocked (Yeo et al., 2008).
Recent studies have shown that growth differentiation factor 9 (GDF9) and bone morphogenetic protein 15 (BMP15; also called GDF9B) play critical roles in oocytes and subsequent embryo developmental competence in several animal species (Gilchrist et al., 2008; Hussein et al., 2006). However, the functions of BMP6 in oocytes are still emerging. BMP6, similar to GDF9 and BMP15, belongs to the transforming growth factor-β (TGF-β) superfamily (Shi and Massagué, 2003) and is a crucial OSF (Paradis et al., 2009; Shi et al., 2009; Sugiura et al., 2010).
Extensive studies have already been conducted to determine the in vivo effects of recombinant BMP6 on GCs and CC (Campbell et al., 2009; Shi et al., 2009; Sugiura et al., 2010). BMP6 physiologically regulates ovarian functions in sheep; it also directs the ovarian infusion of BMP6 that in turn results in acute stimulation of ovarian inhibin A, A4, and estradiol secretion (Campbell et al., 2009). To date, very few studies have investigated BMP6 effects on mammalian oocytes and cloned embryos, especially for yaks. Therefore, we aimed to study the addition of recombinant BMP6 to the oocyte maturation medium and investigated whether it could enhance the developmental potential of yak oocytes and subsequent development of SCNT embryos.
Materials and Methods
All experimental procedures and methods were followed and supervised by the Animal Care Commission of the College of Veterinary Medicine, Gansu Agricultural University. Yak ovaries were obtained from a local slaughterhouse, the Lejiawan Abattoir, located in Xining, China.
All chemicals and reagents were provided by Sigma Aldrich (St. Louis, MO, USA) unless otherwise specified. Disposable, sterile plasticware was purchased from Nunclon (Roskilde, Denmark).
Yak oocytes collection and IVM
The yak ovaries were collected immediately from a commercial abattoir and transported to laboratory in sterile saline at 30–35°C using a thermos flask within 4–6 h. Collected ovaries were rinsed three times with sterile saline at 30–35°C; follicular fluid was aspirated with an 18-gauge needle that was adapted to a 10-mL disposable syringe. COCs were recovered, and only oocytes surrounded by compact cumulus investment and with homogeneous cytoplasm were selected for the culture.
Phosphate-buffered saline (PBS) containing 3% (vol/vol) fetal bovine serum (FBS) was used to wash the yak oocytes three times within 30 min. The maturation medium was composed of Tissue Culture Medium-199 (TCM-199) plus 10% FBS (Sterile Fetal Bovine Serum for Cell Culture, Medicorp, Montreal, QC, Canada), and 200 mM pyruvate, 50 mg/mL gentamicin, 1 μg/mL 17β-estradiol, and 100 μg/mL follicle-stimulating hormone (FSH; Gonal-f, Serono Canada, Inc.). Approximately 50 COCs were cultured in 400 μL of maturation medium in four-well dishes (Nunc, Roskilde, Denmark) and cultured with an oil overlay. A portion of yak COCs were selected and matured in vitro for IVF. The remaining yak COCs were matured with 0, 50, 100, and 200 ng/mL BMP6 in oocyte maturation medium for 24 h at 37°C in 5% CO2 in ambient humidified air and subjected to SCNT.
SCNT and embryo culture
Ear skin of a newborn female yak was used as to establish the nuclear donor cell cultures as previously described (Liu et al., 2012). SCNT, fusion and activation of reconstructed yak oocytes, and cloned embryo cultures were performed using methods described in previous studies (Su et al., 2012, 2014a, b). Briefly, after 24 h of IVM, the COCs were denuded of the cumulus cells by treating with the COCs with 0.1% bovine testicular hyaluronidase. Oocytes with an extruded first polar body were defined as matured and then used for SCNT to produce the cloned yak embryos. The first polar body and a small amount of surrounding cytoplasm in COCs were enucleated using a glass pipette with 15- to 20-μm inner diameter in 100-μL PBS microdrops containing 7.5 μg/mL cytochalasin B and 10% FBS under mineral oil. After enucleation, the same glass pipette was used to inject a single donor cell into the perivitelline space of an enucleated oocyte.
For oocyte–cell fusion, a double electrical pulse of 35V for 10 μsec was applied using a pair of platinum electrodes connected to a micromanipulator. After 2 h in G1.5 medium (Vitrolife AB, Gothenburg, Sweden) containing 5 μg/mL cytochalasin B, 5 μM ionomycin (3–5 min), and 2.0 mM dimethylaminopyridine (4–6 h) were used to activate the reconstructed embryos. After the cloned yak embryos were activated, they were transferred into the G1.5/G2.5 sequential media for further culturing. Thereafter, the droplets of 100 μL of G1.5 medium were prepared in a 60-mm cell culture dish and covered with oil; these droplets were then equilibrated for 2–4 h at 38.5°C in 5% CO2 in ambient humidified air prior to transferring the yak embryos (20–25 embryos/microdrop).
Cleavage embryos were transferred from G1.5 medium to G2.5 droplets and covered with paraffin oil after the third day of culture. Development of SCNT embryos to the two- to four-cell and early stage of blastocyst was observed at 48 h and 168 h of culture, respectively. (The time of embryos transferred into the G1.5 was considered as 0 h.) Six replicates were performed, and approximately 40 COCs were used as per replicate for each group.
In vitro fertilization
IVF was performed as described previously with some modifications (Wang et al., 2011). Briefly, semen in straws was thawed and washed in sperm-Tyrode's albumin lactate pyruvate (Sp-TALP) medium (Parrish et al., 1988). The sperm were centrifuged twice at 500 × g for 10 min and incubated for 1 h for capacitation in Sp-TALP medium, A 100-μL sperm suspension (4 × 106 sperm/mL) was added to approximately 40 COCs in 400 μL of TALP medium in four-well dishes and coincubated for 18–20 h at 37°C in 5% CO2 in ambient humidified air. After the IVF, the presumptive zygotes were liberated from the surrounding cumulus cells and redundant spermatozoa by the vortexing in Dulbecco's phosphate-buffered saline (D-PBS) that contained 0.5% polyvinylpyrrolidone (PVP) and 0.1% hyaluronidase. After washing three times with G1.5 medium, the zygotes were transferred into 100-μL drops of G1.5 medium at 37°C in 5% CO2 in ambient humidified air.
Droplets of 100 μL of the G1.5 medium were prepared in a 60-mm cell culture dish, covered with paraffin oil, and equilibrated for 2–4 h prior to loading of zygotes (20–25 embryos/microdrop). Cleavage embryos were transferred from G1.5 medium to G2.5 droplets and covered with paraffin oil after the third day of culture. IVF embryo development to the two- to four-cell and early blastocyst stage was observed at 48 h and 168 h of culture, respectively (time of embryos transferred into G1.5 was considered as 0 h). Six replicates were performed, and approximately 40 COCs were used per replicate for each group.
Immunofluorescence staining
Embryos were stained immunofluorescently as described previously (Su et al., 2011, 2014a, b) with some modifications. Embryos were fixed in 4% paraformaldehyde in PBS supplemented with 0.5% PVP at 4°C for 12 h. They were permeabilized (0.1% Triton X-100 for 1 h), blocked in blocking solution [5% bovine serum albumin (BSA) in PBS] for 1 h at room temperature, and incubated with primary antibodies against CDX2 (1:200, BioGenex, San Ramon, CA, USA), anti-AcH3K9 (1:500; Abcam, Cambridge, MA, USA), anti-H3K18ac (1:500; Abcam), or anti-H3K9me3 (1:500; Abcam) for 12–18 h at 4°C. A secondary antibody, goat anti-mouse immunoglobulin G (IgG; Bioss, Beijing, China) labeled with Alexa Fluor 555, stained for CDX2. For H3K9ac, H3K18ac, and H3K9me3 staining, Alexa Fluor 488-labeled goat anti-rabbit IgG (Bioss, Beijing, China) was used.
The blastocysts were transferred into PBS with 5 μg/mL 4′,6-diamidino-2-phenylindole (DAPI; Beyotime, Haimen, Jiangsu, China) staining for nuclear DNA. When mounting embryos onto glass slides with a drop of the Antifade Mounting Medium (Beyotime) was done, a Nikon Eclipse Ti-S microscope equipped with a 198 Nikon DS-Ri1 digital camera (Nikon, Tokyo, Japan) was used to analyze the immunofluorescence.
The total number of cells per blastocyst, trophoblast cells [trophectoderm (TE)], and inner cell mass (ICM) cells were counted in four groups to assess the quality of the yak blastocysts. DAPI was used to estimate the total number of blastocyst cells. CDX2 immunostaining was used to assess the number of TE cells. The total number of the nuclei minus the number of TE nuclei was the number of the ICM cells (Su et al., 2011, 2014b). We repeated the experiments three times, and 10 embryos were taken and processed from each replicate.
Quantification of signal intensity
To determine global the levels of H3K9ac, H3K18ac, and H3K9me3 (green fluorescence), two-cell embryos and blastocysts were collected and stained with green fluorescence. Image-Pro Plus software (v. 6.0, Media Cybernetics, Silver Spring, MD, USA) was used to quantify the signal intensities of H3K9ac, H3K18ac, and H3K9me3 fluorescence as previously described (Su et al., 2012, 2014b). The color images were converted to grayscale images using Image-Pro Plus. Acquired micrographs were again changed into grayscale images and detected. The nucleus of the embryo was outlined and considered as the region of interest after optical density was calibrated. The integrated optical density in each area was detected, and the ratio of total optical density in per unit area was calculated for evaluating the average normalized fluorescence intensity of the single embryo. To calculate normalized levels of H3K9ac, H3K18ac, and H3K9me3, we divided them by total DNA content (total DAPI fluorescence intensity). To reduce the differences among embryos, parameters and adjustments of the microscope and exposure times were kept the same while the Image-Pro Plus software was being used for image acquisition and analysis.
We repeated the experiments at least three times with the 10 embryos in each replicate. The relative levels of H3K9ac, H3K18ac, and H3K9me3 in embryos were expressed as the mean and standard error of the mean (SEM). To quantify fluorescence intensity, the intensity levels within the embryos of SCNT + BMP6 and SCNT groups were expressed relative to the average intensity levels within IVF embryos.
Apoptosis of embryos detected by Bax and Bcl-2 gene expression
Fifteen embryos at day 7 of the blastocyst stage in each group were washed with RNase-free water and collected in RNase-free tubes and then immediately submersed in liquid nitrogen. Thereafter, we extracted total RNA from blastocysts using an RNeasy Micro Kit (Quiagen, Valencia, CA, USA) according to the instructions of manufacturer. A Superscript™ III First Strand Synthesis Kit (Invitrogen, Chicago, IL, USA) was used to synthesize the first strand. Samples were kept in a freezer (−20°C) until further use.
Detection of mRNA levels was performed using a Real-Time PCR Detection System (ABI ViiA™ 7; Applied Biosystems, Foster City, CA, USA). Reactions consisted of 2 μL of cDNA and 0.5 μL of the forward and reverse gene-specific primers (Table 1), 10 μL of the SYBR Green II master mix, 0.4 μL of ROX (Bio-Rad, Waltham, MA, USA), and 3.6 μL of water in a total volume of 20 μL. Optimal reactions of quantitative real-time PCR were performed with the following thermal cycling conditions: Denaturation at 95°C for 10 sec, 40 cycles of denaturation at 95°C for 10 sec, annealing at an appropriate temperature for 15 sec (Table 1), and extension fluorescence acquisition at 72°C for 10 sec. The qPCR experiments were repeated in least three separate pools for each group, and a reaction without template served as the negative control.
Table 1.
The Primers Used for qRT-PCR Analysis
| Gene | Sequence 5′ to 3′ | Tm (°C) | Fragment size (bp) | GenBank acc. no. |
|---|---|---|---|---|
| Bax | TTTGCTTCAGGGTTTCATC | 59°C | 174 | NM173894 |
| CAGCTGCGATCATCCTCT | ||||
| Bcl-2 | CTGCACCTGACGCCCTTCAC | 60°C | 236 | NM001166486 |
| GCGTCCCAGCCTCCGTTGT | ||||
| GAPDH | AACCACGAGAAGTATAACAACACC | 59°C | 114 | EU195062 |
| TCCCTCCACGATGCCAAA |
Melting point curves were constructed for each primer after amplification to determine the qPCR product identity. PCR product sizes were detected by the gel electrophoresis on a standard 1.5% agarose gel stained with the ethidium bromide, and detected under ultraviolet light. The relative transcript abundance of the glyceraldehyde 3-phosphate dehydrogenase (GAPDH) gene was used as an endogenous reference. The Ct (threshold cycle) values of the reference gene (GAPDH) among treatments had a coefficient of variance of 1.04%, with an average of 28.73 ± 0.48. The relative quantification of gene expression was performed by the comparative Ct method (Pfaffl et al., 2002), using the IVF day 7 blastocysts as a control sample. The data are shown as relative n-fold differences in relation to the control sample. We repeated experiments for at least three times, and 15 day 7 blastocysts were used in each group.
Statistical Analysis
The data analysis was done by using one-way analysis of variance (ANOVA), and means were compared by least significant difference (LSD) tests using SPSS 18.0 software (SPSS Inc., Chicago, IL, USA). Only BMP16 intervention in blastocysts at a dose of 100 ng/mL was tested in the apoptosis and histone modification assays. The data are expressed as the mean ± SEM, and the differences with p < 0.05 were considered statistically significant.
Results
Effect of BMP6 on subsequent developmental potential of cloned yak embryos
The yak oocytes were treated with different concentrations of BMP6 during IVM to evaluate whether supplementation of BMP6 during IVM could benefit the development of yak oocytes and subsequent development of yak cloned embryos. Thereafter, we calculated ratios of the maturations, cleavage, and blastocysts of cloned yak embryos cultured in vitro (Table 2). Developmental competence of yak oocytes and subsequent development of yak cloned embryos were significantly improved in the groups that received 100 and 200 ng/mL of BMP6 during IVM (Table 2). The rates of maturation, two-cell embryos, and blastocysts were increased by BMP6 within the dose range of 50–100 ng/mL; no increase was observed with the 200 ng/mL concentration (Table 2). The effect of 100 ng/mL BMP6 on the subsequent developmental of yak IVF embryos was also assessed and used as a reference to evaluate the effect of 100 ng/mL BMP6 on cloned yak embryos (Table 3).
Table 2.
Effect of Different Concentrations of Recombinant Human BMP6 on the Subsequent Developmental Competence of Cloned Yak Embryos
| Groups | No. oocytes | No. (mean ± SEM%) MII oocytes | No. (mean ± SEM%) ≥ two-cell embryos | No. (mean ± SEM%) blastocysts |
|---|---|---|---|---|
| 0 ng/mL | 242 | 160 (66.12 ± 2.04)a | 111 (69.40 ± 1.03)a | 33 (20.63 ± 1.32)a |
| 50 ng/mL | 256 | 172 (67.18 ± 1.52)ab | 121 (70.34 ± 1.14)a | 38 (22.09 ± 1.14)ab |
| 100 ng/mL | 238 | 174 (73.11 ± 1.38)c | 136 (78.16 ± 0.93)b | 45 (28.16 ± 1.67)c |
| 200 ng/mL | 261 | 180 (69.73 ± 1.84)b | 132 (73.32 ± 0.88)c | 40 (22.22 ± 1.02)ab |
Six replicates were performed and approximately 40 cumulus–ooctye complexes (COCs) per replicate were used for each group. Numbers in parentheses were development rates (mean ± SEM%), whereas other numbers were total oocytes or embryos number of six replicates among groups.
Development rates of two-cell embryos and blastocysts were the percent of total metaphase II (MII) oocytes and were monitored at 48 and 168 h of culture, respectively (0 h being the time embryos were transferred to G1.5 medium).
Values with different superscripts within columns are significantly different from each other (p < 0.05).
BMP6, bone morphogenetic protein 6; SEM, standard error of the mean.
Table 3.
Effect of 100 ng/mL Recombinant Human BMP6 on Subsequent Developmental Competence of Yak IVF Embryos
| Groups | No. oocytes | No. (mean ± SEM%) MII oocytes | No. (mean ± SEM%)≥ two-cell embryos | No. (mean ± SEM%) blastocysts |
|---|---|---|---|---|
| 0 ng/mL | 233 | 154 (66.10 ± 1.61)a | 114 (74.03 ± 1.92)a | 38 (24.66 ± 0.94)a |
| 100 ng/mL | 251 | 184 (73.30 ± 2.03)b | 148 (80.43 ± 1.35)b | 61 (33.15 ± 1.06)b |
Six replicates were performed and approximately 40 cumulus–ooctye complexes (COCs) per replicate were used for each group. Numbers in parentheses were development rates (mean ± SEM%), whereas other numbers were total oocytes or embryos number of six replicates among groups.
Development rates of two-cell embryos and blastocysts were the percent of total metaphase II (MII) oocytes and were monitored at 48 h and 168 h of culture, respectively (0 h being the time embryos were transferred to G1.5 medium).
Values with different superscripts within columns are significantly different from each other (p < 0.05).
BMP6, bone morphogenetic protein 6; IVF, in vitro fertilization; SEM, standard error of the mean.
The total numbers of cells in early blastocysts were found to be significantly (p < 0.05) increased, and the ratio of ICM to TE in blastocysts was also significantly higher in the BMP6-supplemented groups compared with the nonsupplementation group (control group) (p < 0.05), which exhibited levels similar to the IVF yak embryos (Table 4).
Table 4.
Characterization of Day 7 Yak Blastocysts From Different Treatments
| Groups | No. blastocysts | Total no. of cells | No. of TE cells | No. of ICM cells | No. of ICM cells:total cell ratio (%) |
|---|---|---|---|---|---|
| SCNT | 42 | 85.24 ± 3.12a | 65.65 ± 5.03a | 19.59 ± 2.17a | 22.93 ± 1.43a |
| SCNT + BMP6 | 36 | 103.36 ± 5.28b | 71.16 ± 3.92b | 32.20 ± 2.61b | 31.21 ± 1.62b |
| IVF | 41 | 105.83 ± 6.03b | 72.69 ± 7.41b | 33.14 ± 3.34b | 31.29 ± 1.28b |
The cell numbers in blastocysts were estimated by counting the total number of nuclei using 4′,6-diamidino-2-phenylindole (DAPI), whereas the number of trophectoderm nuclei was assessed using immunostaining for CDX2. The inner cell mass cell number was estimated as the total number of nuclei minus the number of trophectoderm nuclei. The data are shown as mean ± standard error of the mean (SEM). n refers to the total number of blastocysts analyzed.
Values with different superscripts within columns are significantly different from each other (p < 0.05).
TE, trophectoderm; ICM, inner cell mass; BMP6, bone morphogenetic protein 6; SCNT, somatic cell nuclear transfer, IVF, in vitro fertilization.
Effect of BMP6 on epigenetic modifications of yak cloned embryos
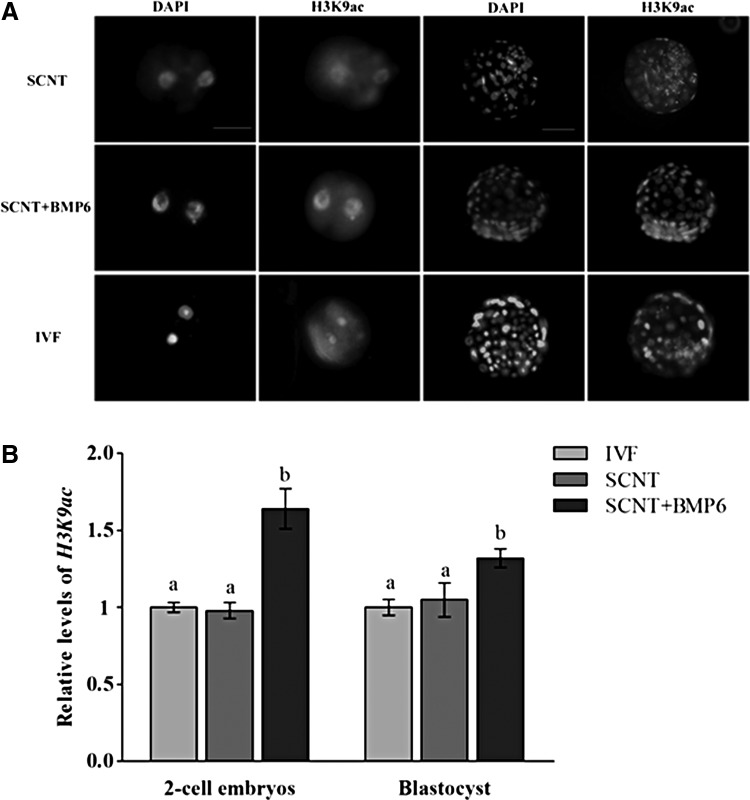
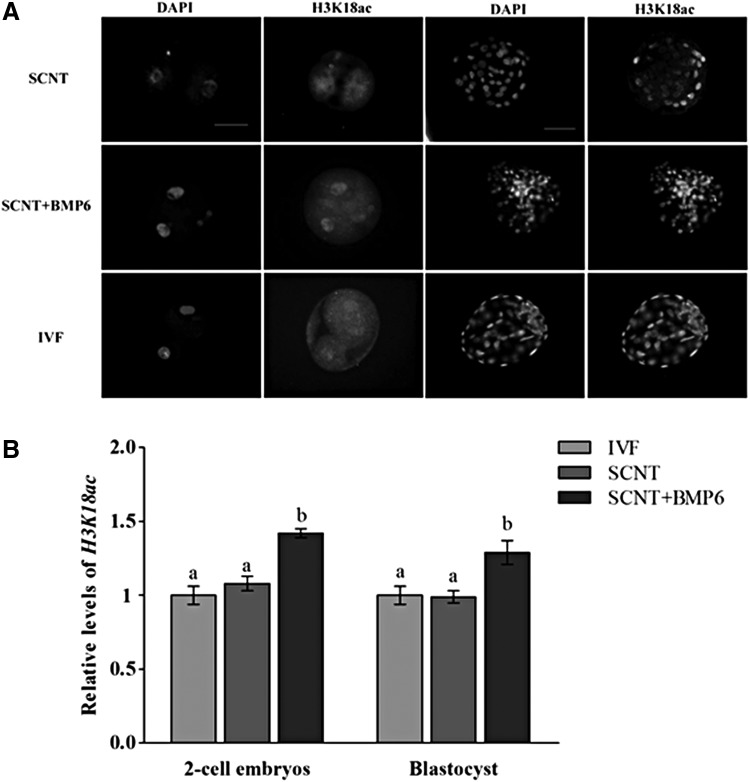
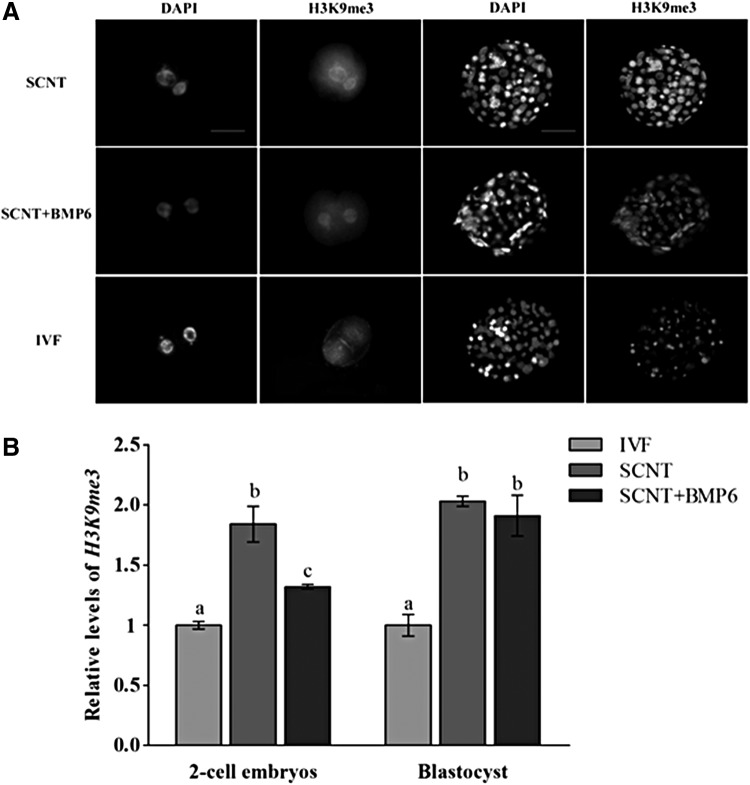
Global levels of acetylated H3K9ac (Fig. 1A, B) and H3K18ac (Fig. 2A, B) and histone H3 trimethylation at lysine 9 (H3K9me3) were analyzed in embryos of two-cell and blastocyst stages (Fig. 3A, B). The levels of H3K9ac in the two-cell and blastocyst stages in SCNT + BMP6 groups were higher than levels in the SCNT group (p < 0.05) (Fig. 1A, B), which is similar to the levels of yak IVF embryos (Fig. 1A, B). The H3K18ac levels in cloned yak embryos of two-cell and blastocyst stages from SCNT + BMP6 groups were also higher compared to both in embryos of same stages from SCNT and IVF groups (p < 0.05) (Fig. 2A, B). The levels in all groups of yak cloned embryos was higher than those in IVF embryos (p < 0.05) (Fig. 3A, B), and H3K9me3 levels in embryos of SCNT group were higher than the levels in SCNT + BMP6 groups, especially at the two-cell stages (Fig. 3A, B). No immunofluorescence was found in embryos incubated without primary or secondary antibodies, which indicated the specificity of methods.
FIG. 1.
Quantification of H3K9ac/DNA signal intensities in embryos at the two-cell and blastocyst stages. (A) Staining of H3K9ac in embryos at the two-cell (left columns) and blastocyst stages (right columns). Each sample was counterstained with DAPI to visualize the DNA. Scale bars, 50 μm. (B) Labeling intensity was expressed relative to the IVF group (set as 1.0). Quantification of the H3K9ac/DNA ratio is represented as the mean ± standard error of the mean. Values with different superscripts differ significantly (p < 0.05). Experiments were replicated three times. In each replication, n = 10 per group.
FIG. 2.
Quantification of H3K18ac/DNA signal intensities in embryos at the two-cell and blastocyst stages. (A) Staining of H3K18ac in embryos at the two-cell (left columns) and blastocyst stages (right columns). Each sample was counterstained with DAPI to visualize the DNA. Scale bars, 50 μm. (B) Labeling intensity was expressed relative to the IVF group (set as 1.0). Quantification of the H3K18ac/DNA ratio is represented as the mean ± standard error of the mean. Values with different superscripts differ significantly (p < 0.05). Experiments were replicated three times. In each replication, n = 10 per group.
FIG. 3.
Quantification of H3K9me3/DNA signal intensities in embryos at the two-cell and blastocyst stages. (A) Staining of H3K9me3 in embryos at the two-cell (left columns) and blastocyst stages (right columns). Each sample was counterstained with DAPI to visualize the DNA. Scale bars, 50 μm. (B) Labeling intensity was expressed relative to the IVF group (set as 1.0). Quantification of the H3K9me3/DNA ratio is represented as the mean ± standard error of the mean. Values with different superscripts differ significantly (p < 0.05). Experiments were replicated three times. In each replication, n = 10 per group.
Effect of BMP6 on apoptosis of blastocysts
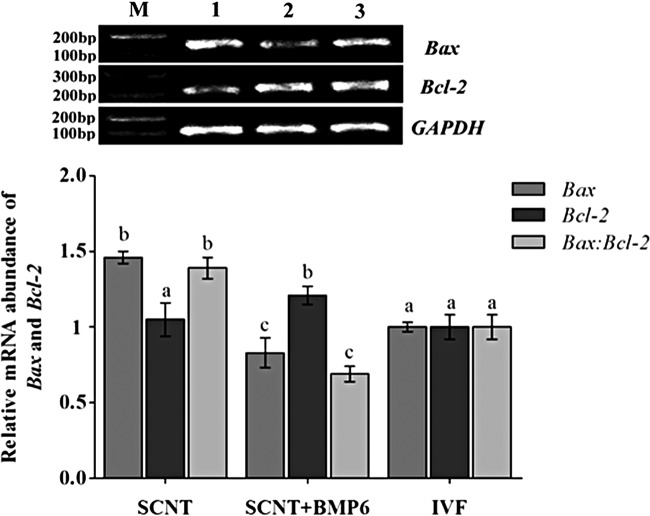
In this study, the apoptotic index in blastocysts of cloned yak embryos was analyzed by detecting expression of the Bax and Bcl-2 genes in the groups treated with 0 and 100 ng/mL BMP6 and the IVF embryos. The genes were selected on the basis of results of our previous study. The relative transcript abundance of the Bax and Bcl-2 genes determined by real-time PCR is shown in Figure 4. The expression level of Bax was significantly lower in the SCNT + BMP6 groups (p < 0.05) (Fig. 4); the level of the Bcl-2 gene was highest in the same group, and the rates of Bax to Bcl-2 were lowest in the SCNT + BMP6 group (Fig. 4). Furthermore, expression levels of Bax and Bcl-2 genes were found not to be significant between SCNT and IVF groups (p > 0.05; Fig. 4), indicating that apoptosis in blastocysts in the SCNT + BMP6 groups was less than that in the IVF or control groups.
FIG. 4.
The mRNA expression of Bax and Bcl-2 in yak day 7 blastocysts. (A) The mRNA expression of Bax and Bcl-2 in yak day 7 blastocysts detected by RT-PCR in the three groups. Lane M, Marker; lane 1, SCNT; lane 2, SCNT+BMP6; lane 3, IVF. Experiments were replicated three times, and 15 day 7 blastocysts were included in each group. (B) Relative expression (mean ± SEM) of Bax and Bcl-2 mRNA in yak day 7 blastocysts and the ratio of Bax to Bcl-2 in each group. The expression levels were shown as a relative quantitation and were analyzed using real-time PCR. GAPDH was used to normalize each gene, and the blastocysts in the IVF group were used as the calibrators. Different letters on the bars indicate values that differ significantly (p < 0.05). Experiments were replicated three times, and 15 day 7 blastocysts were included in each group.
Discussion
Oocyte-secreted paracrine factors, including GDF9, BMP15, and BMP6, play crucial roles in modulating CC functions, such as proliferation, steroidogenesis, DNA synthesis, differentiation, apoptosis, and cumulus expansion (Eppig 2001; Eppig et al., 2005; Shi et al., 2009; Sugiura et al., 2010; Wang et al., 2013). BMP6 increases the viable cell numbers through either mitogenic effects or by increasing cell survival. BMP6 also regulates the generation of activin-A, inhibin-A and follistatin in vivo (Gilchrist et al., 2004). However, the roles of BMP6 on oocytes during IVM have not been investigated, and its effects on the developmental potential of mammalian cloned embryos is still unclear. Here we have provided evidence that BMP6 enhances the developmental potential of oocytes and subsequent development of embryos during IVM.
We demonstrated that the maturation rate and subsequent developmental potential of yak oocytes could be improved with supplementation of BMP6 during IVM (Table 2). These studies suggest that BMP6, similar to GDF9 and BMP15, is also an important OSF that significantly affects the developmental potential of oocytes and subsequent embryos. In addition to enhancing the yield of blastocysts, the quality of blastocysts, including total number of cells, TE and ICM cells in blastocysts, and ratio of ICM to TE, were also increased when the cultures were supplemented with BMP6. These findings are in accordance with the results shown in a similar study using day 8 bovine IVF blastocysts and cloned blastocysts to investigate the effects of other OSFs on blastocyst quality (Hussein et al., 2006; Hussein et al., 2011; Su et al. 2014a, b). These results demonstrate that the quality of blastocysts is also enhanced by BMP6, resulting in biological functions equivalent to GDF9 and BMP15.
Apoptosis is usually detected to evaluate blastocyst quality, and less apoptosis is related to higher success rates (Hardy 1997; Hussein et al., 2005). During the course of apoptosis, anti-apoptotic Bcl-2 and the pro-apoptotic Bax provide a signaling pathway that helps to maintain balance in the cell. The relative levels of these two groups of proteins determine whether the cells survive or undergo apoptosis (Korsmeyer, 1999). Therefore, we detected the Bax and Bcl-2 mRNA levels to evaluate yak blastocyst apoptosis. The results suggested that BMP inhibited cloned blastocyst apoptosis, which was consistent with the effects of GDF9 on cloned bovine embryos (Su et al., 2014a). We also demonstrated that BMP-inhibited apoptosis could be achieved by modulating expression of Bax and Bcl-2.
Cloning efficiency of mammalian might be reduced by aberrant epigenetic nuclear reprogramming of the donor nucleus by maturation oocytes (Su et al., 2014a, b). The roles of histone acetylation and histone methylation, which ultimately influence the development competence of SCNT embryos, should not be neglected during SCNT (Dai et al., 2010; Das et al., 2010; Li et al., 2008; Wee et al., 2007; Yamanaka et al., 2009). Therefore, global levels of H3K9ac, H3K18ac, and H3K9me3 in yak cloned embryos at the two-cell and blastocyst stages were investigated. Results indicated that BMP6 could enhance the acetylation levels of H3K9ac and H3K18ac of yak cloned embryos. Histone hyperacetylation is an important process of nuclear reprogramming that alleviates transcriptional repression and facilitates the formation of recombinant nucleosomes and chromatin (Jones et al., 1998; Nan et al., 1998). Su et al. demonstrated that the addition of GDF9 during IVM improved developmental potential of bovine oocytes; nuclear reprogramming competence and subsequent development were also enhanced by GDF9, which can be related to improvements in cloned embryo development (Su et al., 2014a). In our study, the same results were observed when BMP6 was added during yak oocyte IVM, indicating that the roles of BMP6 during mammalian oocyte IVM are similar to the roles of GDF9.
Histone methylation, such as H3K4me2/3, is another key histone modification and is linked with active transcription (Santos-Rosa et al., 2002) and gene activation in zygotes of mice (Shao et al., 2008) and is significant for transcriptional gene reactivation after oocyte reprogramming (Murata et al., 2010). The H3K9me3 histone methylation mark is associated with transcriptional inhibition of euchromatin, repression of chromatin, and formation of heterochromatin. The levels of H3K9me3 found in yak cloned embryos were higher than that in IVF embryos, indicating that H3K9me3 was reprogrammed aberrantly in yak embryos after SCNT. H3K9me3 levels in embryos from SCNT groups were also higher than the levels in the SCNT + BMP6 groups, especially in the two-cell SCNT embryos, a result that was consistent with the finding in cloned bovine embryos affected by GDF9 (Su et al., 2014a, b). This result indicates that BMP6 can reduce aberrantly reprogrammed cloned embryos, although this phenomenon needs further study.
Yaks (Bos grunniens) are seasonally polyestrous animals, with a breeding season from July to October; calving occurs from April to July. Their reproductive rate is lower (40–60%) than other mammals (Sarkar and Prakash, 2005; Yu and Li, 2001; Zi, 2003). Therefore, it is very important to improve the reproductive efficiency of yaks by techniques of in vitro embryo production and SCNT (Karatzas et al., 1997). OSFs play important roles during oocyte maturation and embryo development (Hussein et al., 2006; Su et al., 2014a, b). In the present study, we found that the supplementation of BMP6 during IVM could improve the developmental competence of oocytes and the subsequent development of cloned yak embryos. Thus, OSFs such as BMP6 can help to improve the efficiency of assisted reproductive technology (ART) for yaks.
Conclusion
In summary, the results presented in this study indicate for the first time that BMP6 can enhance the developmental potential of yak oocytes, similarly to other OSFs; the development of subsequent yak SCNT embryo is also improved. This evidence is conducive to improving the efficiency of yak ART and supports the role of OSFs secreted by oocytes, which could be used to evaluate developmental competence.
Acknowledgments
This study was supported by the National Natural Science Foundation of China (grant nos. 31472244 and 31272616) and the Fostering Foundation for the Excellent Ph.D. Dissertation of Gansu Agricultural University.
Author Disclosure Statement
The authors declare that no conflicting financial interests exist.
References
- Baguisi A., Behboodi E., Melican D.T., Pollock J.S., Destrempes M.M., Cammuso C., Williams J.L., Nims S.D., Porter C.A., and Midura P. (1999). Production of goats by somatic cell nuclear transfer. Nat. Biotechnol. 17, 456–461 [DOI] [PubMed] [Google Scholar]
- Buccione R., Schroeder A.C., and Eppig J.J. (1990). Interactions between somatic cells and germ cells throughout mammalian oogenesis. Biol. Reprod. 43, 543–547 [DOI] [PubMed] [Google Scholar]
- Campbell B., Kendall N., and Baird D. (2009). Effect of direct ovarian infusion of bone morphogenetic protein 6 (BMP6) on ovarian function in sheep. Biol. Reprod. 81, 1016–1023 [DOI] [PubMed] [Google Scholar]
- Chian R., and Sirard M. (1995). Effects of cumulus cells and follicle‐stimulating hormone during in vitro maturation on parthenogenetic activation of bovine oocytes. Mol. Reprod. Dev. 42, 425–431 [DOI] [PubMed] [Google Scholar]
- Dai X., Hao J., Hou X.-j., Hai T., Fan Y., Yu Y., Jouneau A., Wang L., and Zhou Q. (2010). Somatic nucleus reprogramming is significantly improved by m-carboxycinnamic acid bishydroxamide, a histone deacetylase inhibitor. J. Biol. Chem. 285, 31002–31010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das Z.C., Gupta M.K., Uhm S.J., and Lee H.T. (2010). Increasing histone acetylation of cloned embryos, but not donor cells, by sodium butyrate improves their in vitro development in pigs. Cell. Reprogram. 12, 95–104 [DOI] [PubMed] [Google Scholar]
- Eppig J.J. (2001). Oocyte control of ovarian follicular development and function in mammals. Reproduction 122, 829–838 [DOI] [PubMed] [Google Scholar]
- Eppig J.J., Pendola F.L., Wigglesworth K., and Pendola J.K. (2005). Mouse oocytes regulate metabolic cooperativity between granulosa cells and oocytes: Amino acid transport. Biol. Reprod. 73, 351–357 [DOI] [PubMed] [Google Scholar]
- Fatehi A., Zeinstra E., Kooij R., Colenbrander B., and Bevers M. (2002). Effect of cumulus cell removal of in vitro matured bovine oocytes prior to in vitro fertilization on subsequent cleavage rate. Theriogenology 57, 1347–1355 [DOI] [PubMed] [Google Scholar]
- Gilchrist R., Ritter L., and Armstrong D. (2004). Oocyte–somatic cell interactions during follicle development in mammals. Anim. Reprod. Sci. 82, 431–446 [DOI] [PubMed] [Google Scholar]
- Gilchrist R.B., Lane M., and Thompson J.G. (2008). Oocyte-secreted factors: Regulators of cumulus cell function and oocyte quality. Hum. Reprod. Update 14, 159–177 [DOI] [PubMed] [Google Scholar]
- Hardy K. (1997). Cell death in the mammalian blastocyst. Mol. Hum. Reprod. 3, 919–925 [DOI] [PubMed] [Google Scholar]
- Hussein T.S., Froiland D.A., Amato F., Thompson J.G., and Gilchrist R.B. (2005). Oocytes prevent cumulus cell apoptosis by maintaining a morphogenic paracrine gradient of bone morphogenetic proteins. J. Cell Sci. 118, 5257–5268 [DOI] [PubMed] [Google Scholar]
- Hussein T.S., Thompson J.G., and Gilchrist R.B. (2006). Oocyte-secreted factors enhance oocyte developmental competence. Dev. Biol. 296, 514–521 [DOI] [PubMed] [Google Scholar]
- Hussein T.S., Sutton M.D., Melanie L., Gilchrist R.B., and Thompson J.G. (2011). Temporal effects of exogenous oocyte-secreted factors on bovine oocyte developmental competence during IVM. Reprod. Fertil. Dev. 23, 576–584 [DOI] [PubMed] [Google Scholar]
- Jones P.L., Veenstra G.J., Wade P.A., Vermaak D., Kass S.U., Landsberger N., Strouboulis J., and Wolffe A.P. (1998). Methylated DNA and MeCP2 recruit histone deacetylase to repress transcription. Nat. Genet. 19, 187–191 [DOI] [PubMed] [Google Scholar]
- Karatzas G., Karagiannidis A., Varsakeli S., and Brikas P. (1997). Fertility of fresh and frozen-thawed goat semen during the nonbreeding season. Theriogenology 48, 1049–1059 [DOI] [PubMed] [Google Scholar]
- Kato Y., Tani T., Sotomaru Y., Kurokawa K., Kato J.-y., Doguchi H., Yasue H., and Tsunoda Y. (1998). Eight calves cloned from somatic cells of a single adult. Science 282, 2095–2098 [DOI] [PubMed] [Google Scholar]
- Korsmeyer S.J. (1999). BCL-1 gene family and the regulation of programmed cell death. Cancer Research 59, 1693–1700 [PubMed] [Google Scholar]
- Lee B.C., Kim M.K., Jang G., Oh H.J., Yuda F., Kim H.J., Shamim M.H., Kim J.J., Kang S.K., and Schatten G. (2005). Dogs cloned from adult somatic cells. Nature 436, 641–641 [DOI] [PubMed] [Google Scholar]
- Li J., Svarcova O., Villemoes K., Kragh P.M., Schmidt M., Bøgh I.B., Zhang Y., Du Y., Lin L., and Purup S. (2008). High in vitro development after somatic cell nuclear transfer and trichostatin A treatment of reconstructed porcine embryos. Theriogenology 70, 800–808 [DOI] [PubMed] [Google Scholar]
- Li Z., Sun X., Chen J., Liu X., Wisely S.M., Zhou Q., Renard J.-P., Leno G.H., and Engelhardt J.F. (2006). Cloned ferrets produced by somatic cell nuclear transfer. Dev. Biol. 293, 439–448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu B., Zhang H., Hao M., and Yu S. (2012). Establishment and characterization of two fetal fibroblast cell lines from the yak. In Vitro Cell. Dev. Biol. Anim. 48, 619–624 [DOI] [PubMed] [Google Scholar]
- Murata K., Kouzarides T., Bannister A.J., and Gurdon J.B. (2010). Histone H3 lysine 4 methylation is associated with the transcriptional reprogramming efficiency of somatic nuclei by oocytes. Epigenetics Chromatin 3, 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nan X., Ng H.-H., Johnson C.A., Laherty C.D., Turner B.M., Eisenman R.N., and Bird A. (1998). Transcriptional repression by the methyl-CpG-binding protein MeCP2 involves a histone deacetylase complex. Nature 393, 386–389 [DOI] [PubMed] [Google Scholar]
- Onishi A., Iwamoto M., Akita T., Mikawa S., Takeda K., Awata T., Hanada H., and Perry A.C. (2000). Pig cloning by microinjection of fetal fibroblast nuclei. Science 289, 1188–1190 [DOI] [PubMed] [Google Scholar]
- Paradis F., Novak S., Murdoch G.K., Dyck M.K., Dixon W.T., and Foxcroft G.R. (2009). Temporal regulation of BMP2, BMP6, BMP15, GDF9, BMPR1A, BMPR1B, BMPR2 and TGFBR1 mRNA expression in the oocyte, granulosa and theca cells of developing preovulatory follicles in the pig. Reproduction 138, 115–129 [DOI] [PubMed] [Google Scholar]
- Parrish J.J., Susko-Parrish J., Winer M.A., and First N.L. (1988). Capacitation of bovine sperm by heparin. Biol. Reprod. 38, 1171–1180 [DOI] [PubMed] [Google Scholar]
- Pfaffl M.W., Horgan G.W., and Dempfle L. (2002). Relative expression software tool (REST©) for group-wise comparison and statistical analysis of relative expression results in real-time PCR. Nucleic Acids Res. 30, e36–e36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos-Rosa H., Schneider R., Bannister A.J., Sherriff J., Bernstein B.E., Emre N.T., Schreiber S.L., Mellor J., and Kouzarides T. (2002). Active genes are tri-methylated at K4 of histone H3. Nature 419, 407–411 [DOI] [PubMed] [Google Scholar]
- Sarkar M., and Prakash B.S. (2005). Circadian variations in plasma concentrations of melatonin and prolactin during breeding and non-breeding seasons in yak (Poephagus grunniens L.). Anim. Reprod. Sci. 90, 149–162 [DOI] [PubMed] [Google Scholar]
- Shao G.-B., Ding H.-M., and Gong A.-H. (2008). Role of histone methylation in zygotic genome activation in the preimplantation mouse embryo. In Vitro Cell. Dev. Biol. Anim. 44, 115–120 [DOI] [PubMed] [Google Scholar]
- Shi J., Yoshino O., Osuga Y., Koga K., Hirota Y., Hirata T., Yano T., Nishii O., and Taketani Y. (2009). Bone morphogenetic protein-6 stimulates gene expression of follicle-stimulating hormone receptor, inhibin/activin β subunits, and anti-Müllerian hormone in human granulosa cells. Fertil. Steril. 92, 1794–1798 [DOI] [PubMed] [Google Scholar]
- Shi Y., and Massagué J. (2003). Mechanisms of TGF-β signaling from cell membrane to the nucleus. Cell 113, 685–700 [DOI] [PubMed] [Google Scholar]
- Su J., Wang Y., Li Y., Li R., Li Q., Wu Y., Quan F., Liu J., Guo Z., and Zhang Y. (2011). Oxamflatin significantly improves nuclear reprogramming, blastocyst quality, and in vitro development of bovine SCNT embryos. PloS One 6, e23805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su J., Wang Y., Li R., Peng H., Hua S., Li Q., Quan F., Guo Z., and Zhang Y. (2012). Oocytes selected using BCB staining enhance nuclear reprogramming and the in vivo development of SCNT embryos in cattle. PloS One 7, e36181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su J., Hu G., Wang Y., Liang D., Gao M., Sun H., and Zhang Y. (2014a). Recombinant human growth differentiation factor-9 improves oocyte reprogramming competence and subsequent development of bovine cloned embryos. Cell. Reprogram. 16, 281–289 [DOI] [PubMed] [Google Scholar]
- Su J., Wang Y., Zhang L., Wang B., Liu J., Luo Y., Guo Z., Quan F., and Zhang Y. (2014b). Oocyte‐secreted factors in oocyte maturation media enhance subsequent development of bovine cloned embryos. Mol. Reprod. Devel. 81, 341–349 [DOI] [PubMed] [Google Scholar]
- Sugiura K., Su Y.-Q., and Eppig J.J. (2010). Does bone morphogenetic protein 6 (BMP6) affect female fertility in the mouse? Biol. Reprod. 83, 997–1004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakayama T., Perry A.C., Zuccotti M., Johnson K.R., and Yanagimachi R. (1998). Full-term development of mice from enucleated oocytes injected with cumulus cell nuclei. Nature 394, 369–374 [DOI] [PubMed] [Google Scholar]
- Wang X.-L., Wang K., Zhao S., Wu Y., Gao H., and Zeng S.-M. (2013). Oocyte-secreted growth differentiation factor 9 inhibits BCL-2-interacting mediator of cell death-extra long expression in porcine cumulus cell. Biol. Reproduction 89, 56. [DOI] [PubMed] [Google Scholar]
- Wang Y., Su J., Wang L., Xu W., Quan F., Liu J., and Zhang Y. (2011). The effects of 5-aza-2′-deoxycytidine and trichostatin A on gene expression and DNA methylation status in cloned bovine blastocysts. Cell. Reprogram. 13, 297–306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wee G., Shim J.-J., Koo D.-B., Chae J.-I., Lee K.-K., and Han Y.-M. (2007). Epigenetic alteration of the donor cells does not recapitulate the reprogramming of DNA methylation in cloned embryos. Reproduction 134, 781–787 [DOI] [PubMed] [Google Scholar]
- Wilmut I., Schnieke A.E., McWhir J., Kind A.J., and Campbell K.H. (1999). Viable offspring derived from fetal and adult mammalian cells. Nature 385, 810–813 [DOI] [PubMed] [Google Scholar]
- Yamanaka K.-i., Sugimura S., Wakai T., Kawahara M., and Sato E. (2009). Acetylation level of histone H3 in early embryonic stages affects subsequent development of miniature pig somatic cell nuclear transfer embryos. J. Reprod. Dev. 55, 638–644 [DOI] [PubMed] [Google Scholar]
- Yang X., Smith S.L., Tian X.C., Lewin H.A., Renard J.-P., and Wakayama T. (2007). Nuclear reprogramming of cloned embryos and its implications for therapeutic cloning. Nat. Genet. 39, 295–302 [DOI] [PubMed] [Google Scholar]
- Yeo C.X., Gilchrist R.B., Thompson J.G., and Lane M. (2008). Exogenous growth differentiation factor 9 in oocyte maturation media enhances subsequent embryo development and fetal viability in mice. Hum. Reprod. 23, 67–73 [DOI] [PubMed] [Google Scholar]
- Yu S.J., and Li F.D. (2001). Profiles of plasma progesterone before and at the onset of puberty in yak heifers. Anim. Reprod. Sci. 65, 67–73 [DOI] [PubMed] [Google Scholar]
- Zhang L., Jiang S., Wozniak P.J., Yang X., and Godke R.A. (1995). Cumulus cell function during bovine oocyte maturation, fertilization, and embryo development in vitro. Mol. Reprod. Dev. 40, 338–344 [DOI] [PubMed] [Google Scholar]
- Zi X.D. (2003). Reproduction in female yaks (Bos grunniens) and opportunities for improvement. Theriogenology 59, 1303–1312 [DOI] [PubMed] [Google Scholar]