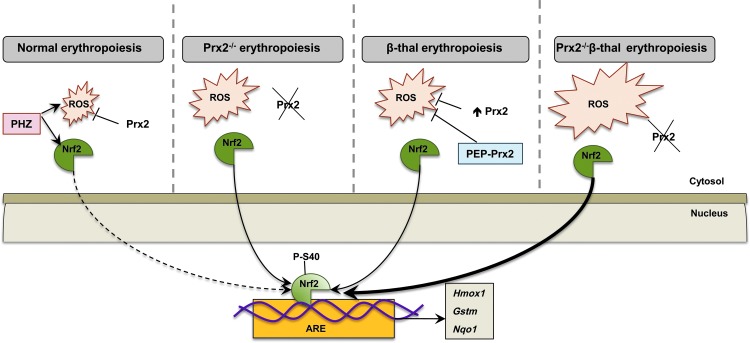
FIG. 7.
Schematic diagram of the working model proposed for Prx2 cooperating with Nrf2 to limit oxidative damage in β-thalassemic erythropoiesis. In WT mice, the physiological generation of ROS during erythropoiesis is controlled by antioxidant systems such as Prx2 and Nrf2 transcriptional factor is inactive. The acute oxidative stress induced by PHZ activates Nrf2, which, in turn, upregulates ARE-genes of antioxidant systems such as HO-1 (Hmox1), Gstm, or nqo1. The absence of Prx2 promotes ineffective erythropoiesis and increases ROS levels with associated activation of Nrf2 as a back-up mechanism to control oxidative stress. In β-thalassemic erythroid precursors, despite the upregulation of Prx2, oxidative stress promotes the activation of Nrf2, which upregulates ARE-genes for antioxidant systems. PEP-Prx2 treatment ameliorates ineffective erythropoiesis and reduced ROS levels. In Prx2−/− Hbbth3/+ mice, the absence of Prx2 worsens β-thalassemic hematological phenotype and ineffective erythropoiesis with a further increase in ROS levels and further activation of Nrf2. Thus, in stress erythropoiesis, Prx2 and Nrf2 act to contain the toxic effects of high ROS levels to ensure cell survival. ARE, antioxidant responsive element; PHZ, phenylhydrazine. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars