Figure 7.
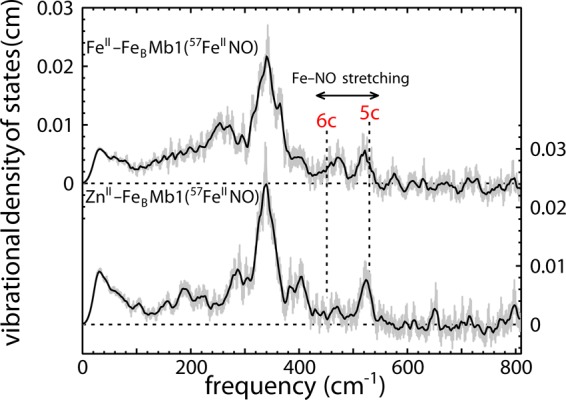
The heme iron VDOS reveals that the presence of a second metal in the nonheme site influences NO binding to FeII-FeBMb1(57FeII) and ZnII-FeBMb1(57FeII). Fe–NO stretching vibrations, clearly resolved above 400 cm–1, probe the axial ligation. For reference, dashed lines indicate Fe–NO stretching frequencies reported for native horse heart MbNO (452 cm–1),93 characteristic of a six-coordinate complex with NO coordinated trans to a histidine ligand, and for Fe(DPIX)(NO) (528 cm–1),88 a typical five-coordinate heme NO complex. A substantial fraction of hemes exhibit an Fe–NO stretching frequency characteristic of five-coordinate heme nitrosyls when either ZnII or FeII is present in the nonheme site. This contrasts with previous measurements on native MbNO,92 which revealed a NRVS signal consistent with six-coordinate heme NO.
