Abstract
Nickel nanoparticles, formed in situ and used in combination with micellar catalysis, catalyze Suzuki Miyaura cross-couplings in water under very mild conditions.
Keywords: Ni-Nanoparticles, Micellar Catalysis, Green Chemistry, E Factor, Suzuki-Miyaura couplings
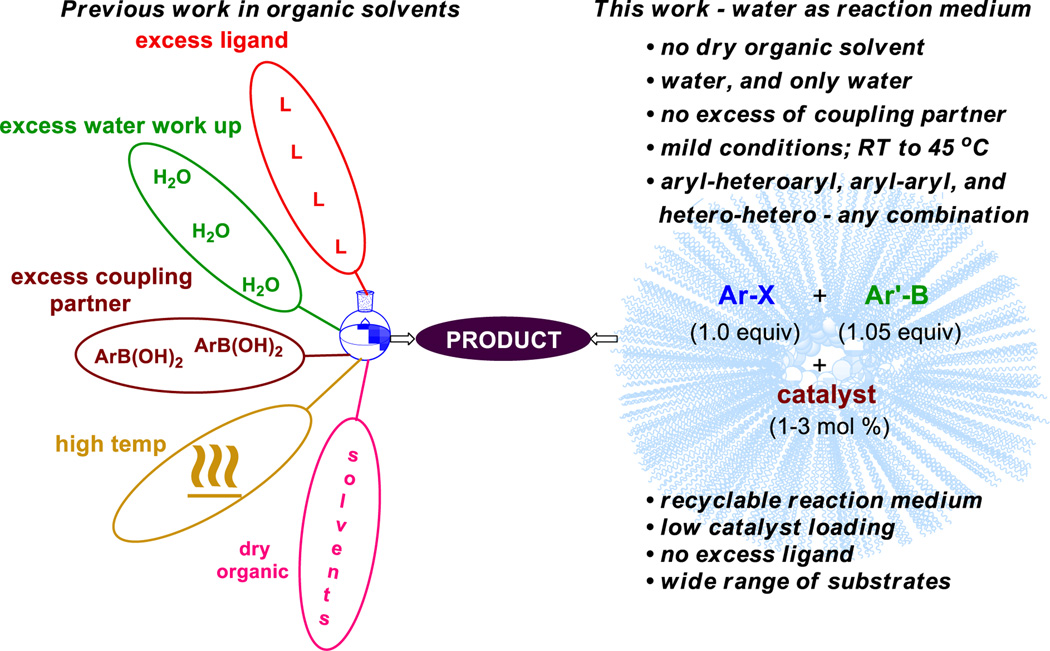
Transition-metal mediated cross-couplings, especially those catalyzed by palladium, have become truly fundamental tools in organic synthesis. Most heavily utilized over the past decade, by far, have been Suzuki-Miyaura (SM) reactions, especially those that provide rapid entry to biaryl frameworks.[1] Over the past 35+ years since this Nobel Prize winning chemistry was introduced, circumstances surrounding use of all such Pd-mediated bond constructions have changed; i.e.; from an environmental perspective, as well as a developing awareness of the finite levels of platinoids accessible by current technologies that are being quickly depleted. The cost of this precious metal is almost always a factor, especially in process research, further encouraging alternative methodologies that accomplish similar goals.[2] The National Science Foundation has already begun to encourage research into less costly and earth-abundant alternative metals.[3]
Beginning in 2008, we introduced several Pd-catalyzed SM processes that take advantage of the hydrophobic effect operating within newly engineered nanoparticles in water. This approach dramatically reduces organic waste associated with use of organic solvents as reaction media.[2] A switch to nickel under such micellar catalysis conditions represents another opportunity to replace a precious metal with its base metal alternative. The virtues of nickel, including its greater nucleophilicity relative to palladium due to its smaller size, and its heightened reactivity towards aromatic chlorides due to the strength of the resulting Ni-Cl bond, might be further accentuated given the high concentrations typically found within nanomicelles.[4] In this report we describe new technology that offers not only the most general solution available for such valued nickel-based cross-couplings, but also a process that advances the capabilities of prior art in this field and is, by far, the most environmentally responsible reported to date (Scheme 1).
Scheme 1.
Nanoparticle approach to Ni-catalyzed SM couplings in water.
Since the initial disclosure by Miyaura in 1996, nickel-catalyzed Suzuki-Miyaura couplings have been extensively investigated.[5] Most literature reports require relatively high reaction temperatures (usually ≥100 °C),[6] are limited to either heteroaryl or homoaryl partners,[7] and involve ligand loadings beyond that of the metal[8] which can poison the catalyst over time.[5c,9] In addition, all are conducted in organic solvents. In the composite, these features tend to drive synthetic chemists back towards reliance on palladium.[1,10] An early report by Genet et al. did focus on a water-soluble nickel catalyst, but the couplings were limited to aryl chlorides, required 10 mol % NiCl2 and 50 mol % ligand loading, as well as significant heating to accommodate water-insoluble coupling partners.[11] More recently, significant advances by Hartwig,[12] Buchwald,[13] Garg,[14] and others[2] provide solutions to some of these limitations, in particular the issues of catalyst stability and sensitivity, loadings, substrate scope, and reproducibility on somewhat larger scales. However, these still all involve organic solvents, an excess of heavy base, elevated temperatures, and importantly, an excess of a coupling partner, while none offers recycling of either the solvent or catalyst.
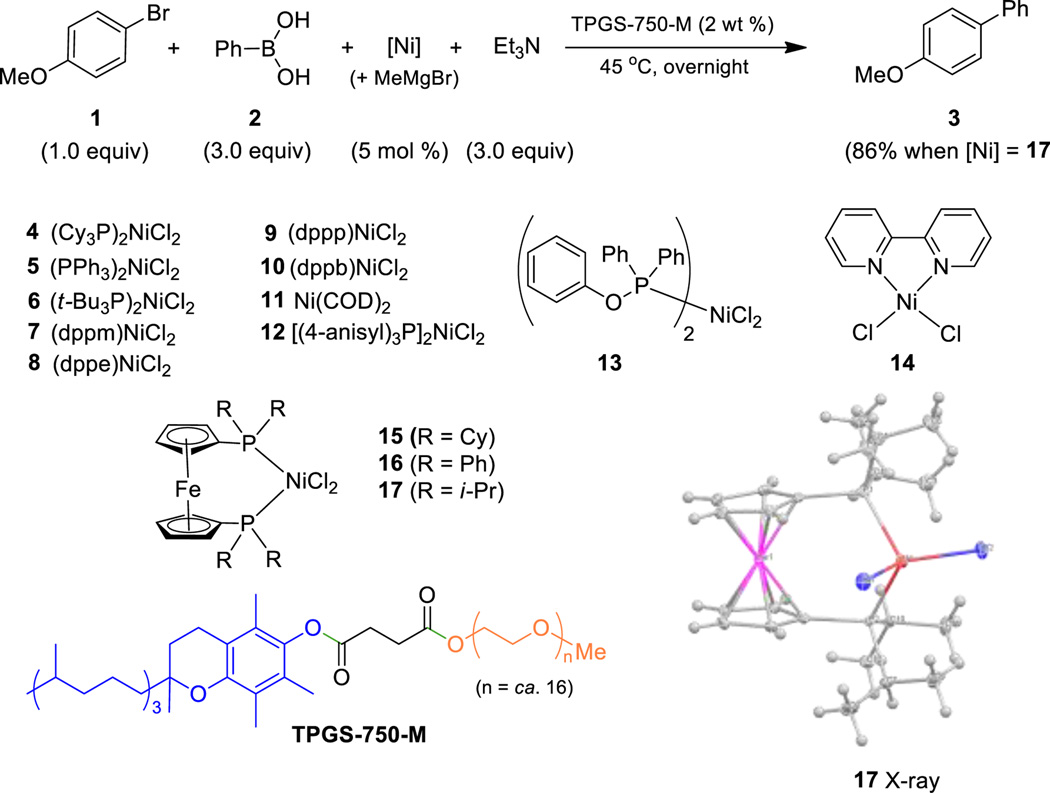
Our study began with the model reaction between aryl bromide 1 and phenylboronic acid 2 using various nickel catalysts, along with triethylamine as base in an aqueous solution containing two weight percent of commercially available designer surfactant TPGS-750-M (Aldrich catalog #733857) at 45 °C. Screening of several catalysts revealed the active nature of species 4, 6, and 14–17 (Scheme 2). Catalyst 17 (see X-ray, below) was found to be the most effective, with biaryl 3 being obtained in 86% isolated yield (see SI for details).
Scheme 2.
Initial screening of nickel catalysts.
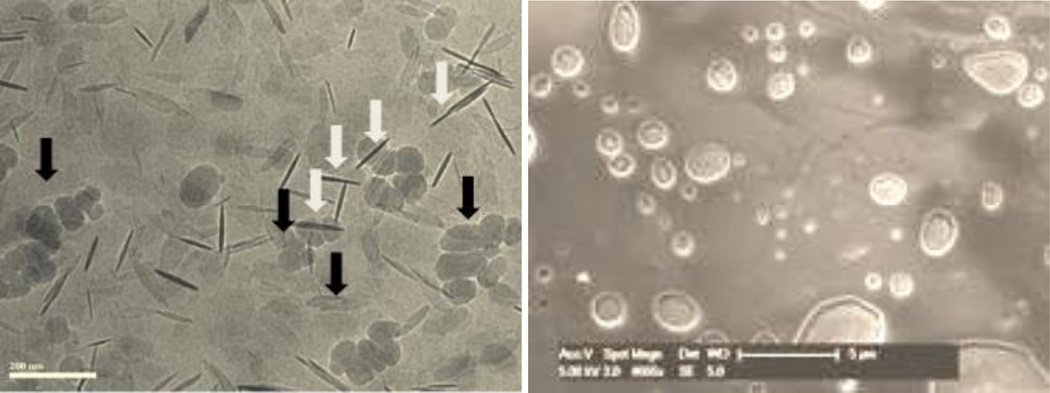
Optimization studies revealed a dependence on several variables, most notably pre-activation of the catalyst using an equivalent of MeMgBr equal to the amount of nickel in THF at room temperature. Other variables included the choice of surfactant, base, and global concentration of the aqueous reaction medium. While optimum conditions rely on ligand 1,1’-bis(diisopropylphosphino)ferrocene (dippf) for aryl-aryl couplings, commercially available (bis)diphenylphosphinoferreocene (dppf) afforded best results for couplings involving heteroaryl partners. Also noteworthy is that only 0.35 equivalents of K3PO4 is needed as base, while previous reports relied on multiple equiv-alents.[6b,12] Moreover, the ratio of aryl boron to aryl halide could be lowered to almost stoichiometric levels (1.05:1). Among the choices between boronic acids or esters, aryl-Bpin esters exhibited greater reactivity over the corresponding boronic acids, allowing for couplings to occur at room temperature (22 °C) in micellar nanoreactors (0.5 M), while reactions of boronic acids required mild heating to 45 °C mainly to increase solubilization due to their highly crystalline nature. Under these conditions, NMR spectroscopy revealed that the boronic acids are stable; hence, the very limited amounts of excess reagent required. Moreover, the solubility of boronic acids was found to be far superior in aqueous TPGS-750-M as compared to that in organic solvents (see SI). Further investigations regarding the nature of the active catalyst revealed the presence of hybrid nickel nanoparticles, confirmed by cryo-TEM (Figure 1, left), SEM (Figure 1, right), and EDX experiments. Nanoparticles were found to be either seemingly inside the micelles (black arrows) or on the surface of aggregated nanomicelles (white arrows), presumably enhancing delivery of educts from the micellar lipophilic cores.
Figure 1.
(left) Cryo-TEM and (right) SEM images of active catalyst in a 3 wt.% aqueous solution of TPGS-750-M (See SI for detailed elemental composition).
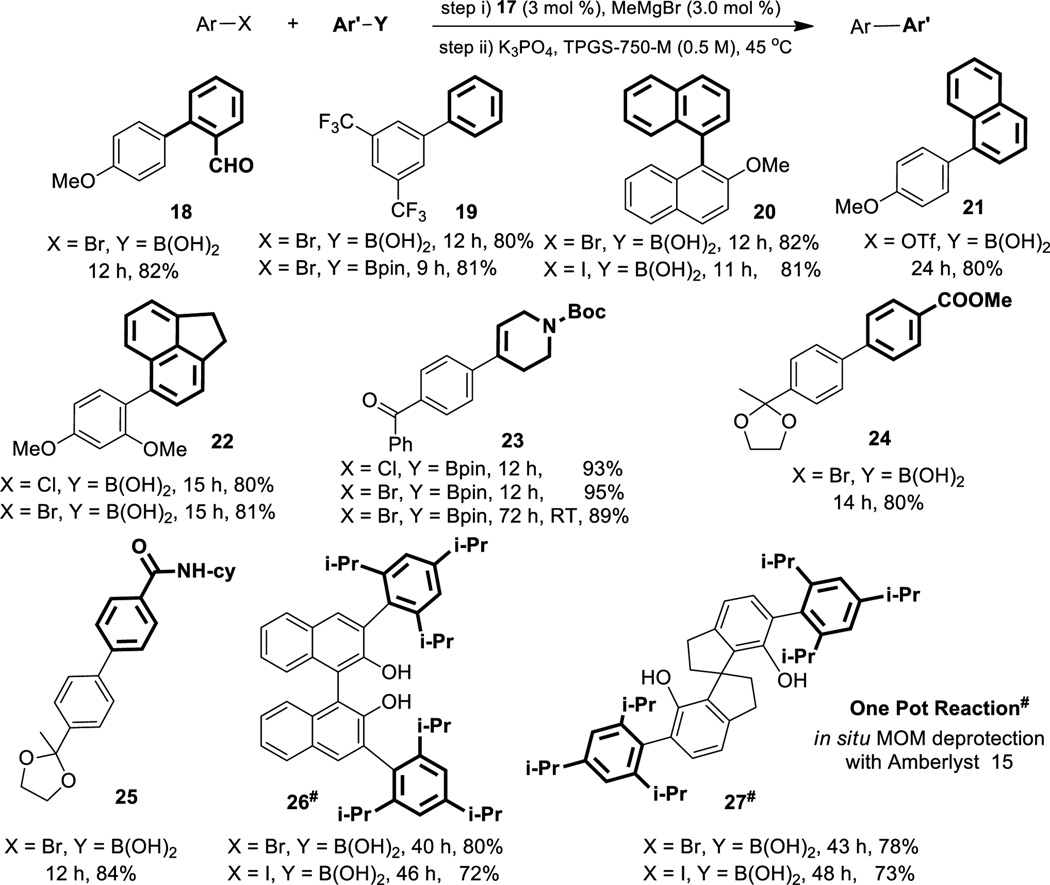
In establishing substrate scope, particular attention was paid to both functional group tolerance and steric issues. As illustrated in Table 1, this technology displays remarkable generality, in contrast to most existing methods, leading to biaryl products independent of the halide leaving group (chlorides, bromides, and iodides). Residues such as formyl (18), trifluoromethyl (19), carbamate (23), ester (24), acetal (24, 25), and amide (25) exerted virtually no influence on these couplings. Cases of notable steric congestion can be found in products 26, and 27. An alkenyl boronate gave highly functionalized styrene 23, generated at either the typical 45 °C or at room temperature, although the latter conditions required an extended reaction time to afford a comparable yield. Cross-couplings between a bis-MOM-protected, 3,3’-dibromo or diiodo-BINOL and a highly hindered boronic acid led after MOM deprotection in a single pot, to the doubly derivatized biaryl 26. Likewise, biaryl 27 could be fashioned using the same 2-step, 1-pot sequence (for additional examples in Table 1, see SI).
Table 1.
Substrate scope for aryl-aryl cross-coupling
Conditions: Aryl-X (0.5 mmol, 1.0 equiv), Aryl-Y (0.53 mmol, 1.05 equiv). Sequence of addition: mixing of 17 with MeMgBr for 1 min at RT under Ar followed by sequential addition of Aryl-X with 30 sec stirring, K3PO4 (0.18 mmol, 0.35 equiv), and Ar-Y. #Aryl-Y (1.05 mmol, 2.1 equiv), 17 (0.03 mmol, 6.0 mol %), MeMgBr (0.03 mmol, 6 mol %), K3PO4 (0.35 mmol, 0.70 equiv), same sequence of addition as with other Ar-X.
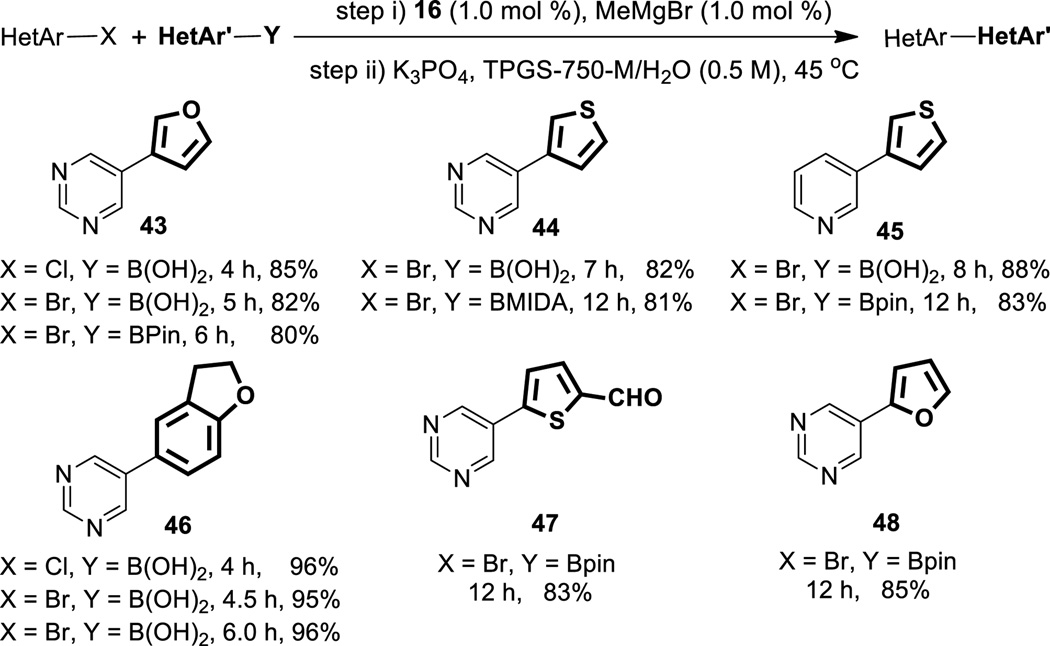
Formation of products reflecting an aromatic/hetero-aromatic combination of partners was next explored (Table 2; see also SI for additional examples). Interestingly, such systems were found to undergo cross-couplings more readily and in higher yields compared to aryl/aryl cases. No reaction was observed with non-ligated NiCl2 as catalyst, suggesting that the heteroaromatic is not likely acting as both educt and ligand. Greater reactivity of pyrimidines as compared to pyridines and thiophenes was noted. Heteroaryl chlorides were typically as, or more, reactive compared to the corresponding bromides and iodides.
Table 2.
Substrate scope for aryl-heteroaryl cross-couplings
Conditions: Ar/HetAr-X (0.5 mmol, 1.0 equiv.), Ar’/HetAr’-Y (0.53 mmol, 1.05 equiv.). Sequence of addition-mixing of 16 with MeMgBr for 1 min at RT under Ar followed by sequential addition of Aryl-X with 30 sec of stirring, K3PO4 (0.18 mmol, 0.35 equiv), and Ar-Y.
Functional group compatibility is equally high in these cases as well, and excellent yields were obtained for products containing dimethylamino (29), methoxy (32, 35, and 39), cyano (34), fluoro (36, 38, and 41), sulfonyl (37), and carbonyl (38, 41) groups. Either catalyst 16 or 17 can be used in these transformations, although 16 is the more active and is commercially available (see SI). Only 1.5–2.0 mol % of 16 is required in these reactions. Boronic acids were found to be slightly more reactive than the corresponding Bpin esters, the opposite to that found in aryl/aryl couplings (vide supra). In the particularly challenging case of 4-pyridylboronic acid, which is rapidly lost to protio-deborylation under SM coupling conditions, use of the corresponding MIDA boronate was successful, leading to biaryl product 34.
Cross-couplings where both partners are heteroaromatics could also be effected, in these cases with an associated drop in catalyst 16 loading to 1 mol % (Table 3; products 43–48). As seen previously with heteroaryl boronic acids, these types of educts are more reactive partners relative to their corresponding Bpin esters and MIDA boronates. Again, heteroaryl chlorides are consistently more reactive than their bromide counterparts.
Table 3.
Hetero-heteroaryl cross-couplings
Conditions: HetAr-X (0.5 mmol, 1.0 equiv), HetAr’-Y (0.53 mmol, 1.05 equiv). Sequence of addition: mixing of 16 with MeMgBr for 1 minute at RT under Ar followed by sequential addition of HetAr-X with 30 sec stirring, K3PO4 (0.18 mmol, 0.35 equiv), and HetAr’-Y.
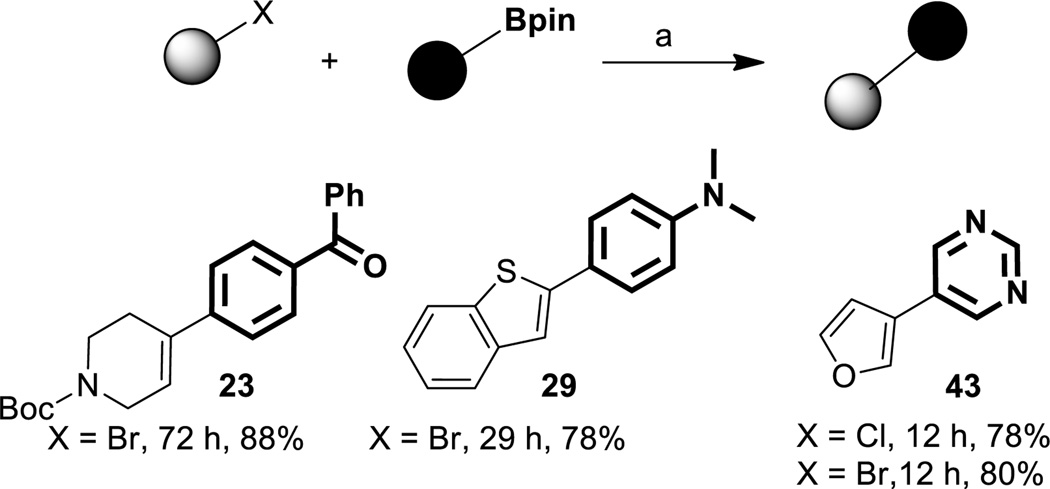
Several examples from the Tables above could be arrived at with essentially equal facility by inverting the nature of the coupling partners. Thus, as illustrated in Table 4 (and in the SI), representative cases involving each of the various types of product biaryls (i.e., aryl-aryl, aryl-heteroaryl, and hetero-heteroaryl) could be prepared, attesting to the inherent flexibility of this very mild and selective Ni-catalyzed coupling approach.
Table 4.
Room temperature cross-couplings with reversal of polarity in coupling partners
Conditions(a): Aryl-X (0.5 mmol, 1.0 equiv), Ar’-Bpin (0.53 mmol, 1.05 equiv). Sequence of addition-mixing of 16 or 17 with MeMgBr (1:1) for 1 min at RT under Ar followed by sequential addition of Aryl-X with 30 sec of stirring, K3PO4 (0.18 mmol, 0.35 equiv), and Ar-Bpin. Note: For Ar-HetAr’ or HetAr-Ar’ systems 1.5 mmol of 16; HetAr-HetAr’: 1.0 mmol of 16, and for Ar-Ar’ systems: 3 mmol 17 was employed as pre-catalyst.
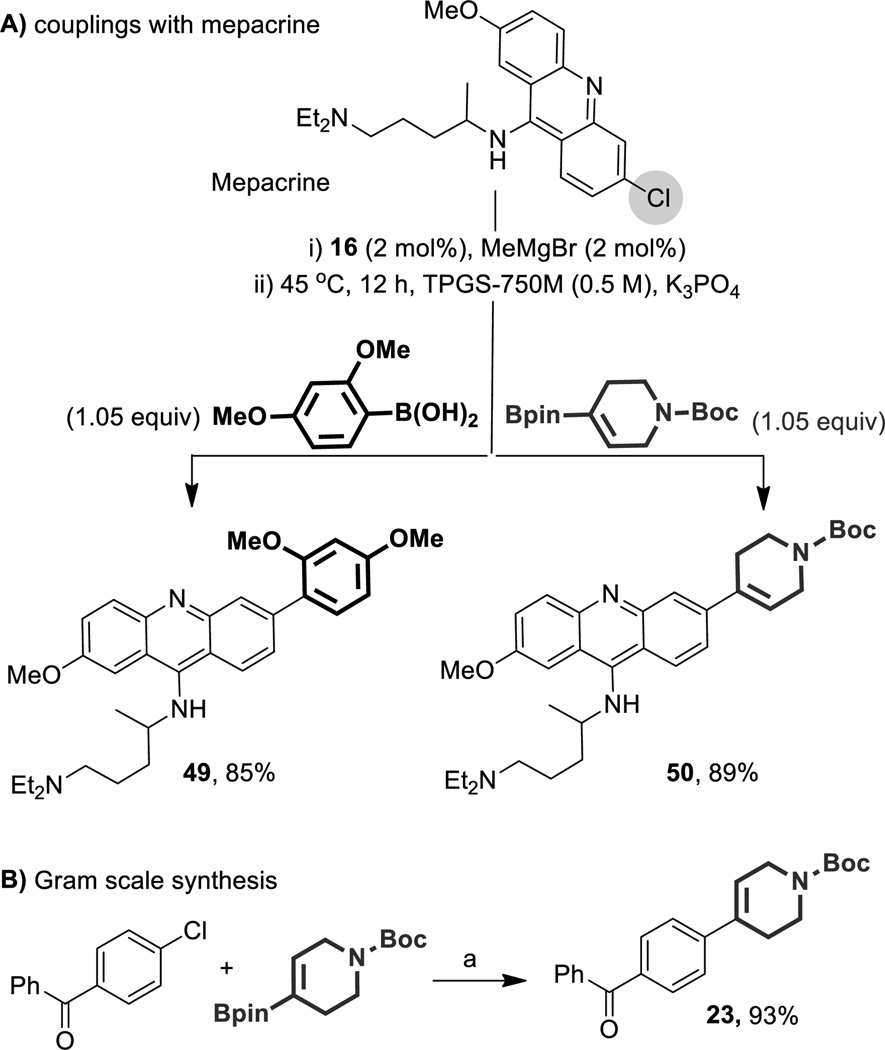
To gauge the prospects for applications to complex synthesis and/or functionalized molecule analog formation, mepacrine, an antiprotozoal, antirheumatic and intrapleural sclerosing agent was selected as a representative coupling partner. Introduction of both aryl and alkenyl groups via displacement from this heteroaromatic chloride took place in excellent yields (Scheme 3, A). The mild conditions typically associated with these milligram scale reactions were also applicable on a one gram scale, affording comparable results (Scheme 3, B).
Scheme 3.
Cross-coupling with mepacrine, and a gram scale reaction. Conditions: (a) Aryl-Cl (0.5 mmol, 1.0 equiv), vinyl-Bpin (0.53 mmol, 1.05 equiv). Sequence of addition-mixing of 16 with MeMgBr for 1 min at RT under Ar followed by sequential addition of Aryl-X with 30 sec of stirring, K3PO4 (0.18 mmol, 0.35 equiv), and Ar-Y.
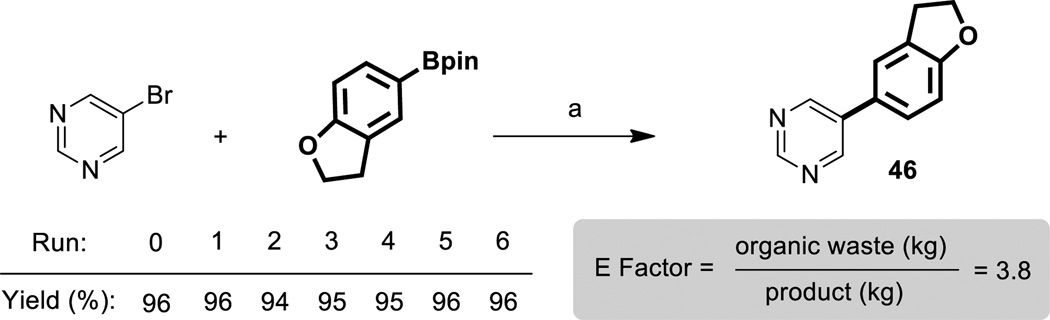
Studies were also conducted to assess the potential for recycling of the reaction medium. Designer surfactants such as TPGS-750-M are engineered to remain in water, while the product undergoes in-flask extraction with minimum amounts of an organic solvent, such as MTBE. For the reaction shown in Scheme 4, product 46 was obtained in consistently high yields over six consecutive recycles. Following each reaction, the product was extracted with MTBE, while the aqueous layer remained in the reaction vessel and was reused for each successive cycle. Small amounts (0.5 mol %) of the nickel catalyst were added after the first four recycles, while 1 mol % was needed for the last two, given the sensitivity of Ni(0) during handling. Calculation of an E Factor[15] based on organic solvent usage as a measure of “greenness” led to a value of only 3.8, which is ca. an order of magnitude below those values typically seen for chemistry done in the fine chemicals and pharmaceuticals arenas.
Scheme 4.
E Factor and recycle studies. a) 5-bromopyrimidine (0.5 mmol, 1.0 equiv), Ar-Bpin (0.53 mmol, 1.05 equiv), 16 (0.0075 mmol, 1.5 mol %), MeMgBr (0.0075 mmol, 1.5 mol %), K3PO4 (0.18 mmol, 0.35 equiv), *TPGS-750-M (3 wt %) 1 mL, 45 °C, 6 h. TPGS-750-M was recycled in all runs. In each recycle, freshly generated active catalyst (0.0075 mmol) and K3PO4 (0.18 mmol) were added (see SI for details).
Another potentially important aspect of this process concerns the amount of nickel that is carried through and into the product from these cross-couplings. This can be a crucial factor governing not only whether, but also at which point such couplings may be utilized, in particular when planning a sequence to an API. ICP analyses of two products isolated using standard “in-flask” extraction and routine flash chromatography afforded products with each retaining ≤5 ppm Ni (see SI).
In summary, Suzuki-Miyaura reactions can now be effected using a new procedure of considerable generality and functional group tolerance that relies on inexpensive nickel in nanoparticle form. The process is enabled by the proper choice of ligand on the metal, matched to the hydrophobic effect characteristic of aqueous nanomicelles. This new technology also features, unlike prior art, greater substrate scope, essentially stoichiometric levels of reaction partners, considerable flexibility in the choice of both leaving group and source of boron, very mild reaction conditions, and notably, the option for recycling of the aqueous medium containing both the surfactant and catalyst.
Supplementary Material
Acknowledgments
Financial support provided by the NIH (GM 86485) is warmly acknowledged. Assistance with physical analyses (AFM and cryo-TEM) provided by Mark Cornish and Stephan Krämer is warmly acknowledged. Technical help by Justin Smith is also much appreciated. We also warmly thank Johnson Matthey (Tom Colacot and co-workers) for providing the initial (DIPF)NiCl2 used in this study, and Dr. John Reilly (Novartis, Cambridge) for obtaining the ICP data.
References
- 1.a) Johansson CCC, Kitching MO, Colacot TJ, Snieckus V. Angew. Chem., Int. Ed. 2012;51:5062. doi: 10.1002/anie.201107017. [DOI] [PubMed] [Google Scholar]; b) Wang N. Youji Huaxue. 2011;31:1319. [Google Scholar]; c) Halford B. Chem. Eng. News. 2010;88:7. [Google Scholar]; d) Lipshutz BH, Isley NA, Fennewald JC, Slack ED. Angew. Chem., Int. Ed. 2013;52:10952. doi: 10.1002/anie.201302020. [DOI] [PMC free article] [PubMed] [Google Scholar]; e) Colacot T. J Platinum Met. Rev. 2009;53:183. [Google Scholar]; f) Colacot T. New Trends in Cross-Couplings: Theory and Applications. RCS Catalysis Series. 2014 can be found under http://pubs.rsc.org/en/content/ebook/9781849738965#!divbookcontent. [Google Scholar]; g) Magano J, Monfette S. ACS Catal. 2015;5:3120. [Google Scholar]
- 2.a) Lipshutz BH, Petersen TB, Abela AR. Org. Lett. 2008;10:1333. doi: 10.1021/ol702714y. [DOI] [PubMed] [Google Scholar]; b) Lipshutz BH, Abela AR. Org. Lett. 2008;10:5329. doi: 10.1021/ol801712e. [DOI] [PubMed] [Google Scholar]; c) Isley NA, Gallou F, Lipshutz BH. J Am. Chem. Soc. 2013;135:17707. doi: 10.1021/ja409663q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.For details, please NSF website, http://www.nsf.gov/pubs/2011/nsf11552/nsf11552.htm.
- 4.a) Tasker SZ, Standley EA, Jamison TF. Nature. 2014;509:299. doi: 10.1038/nature13274. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Handa S, Lippincott DJ, Aue DH, Lipshutz BH. Angew. Chem., Int. Ed. 2014;53:10658. doi: 10.1002/anie.201404729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Saito S, Sakai M, Miyaura N. Tetrahedron Lett. 1996;37:2993. Saito S, Oh-tani S, Miyaura N. J Org. Chem. 1997;62:8024. doi: 10.1021/jo9707848. C) Review: Han F-S. Chem. Soc. Rev. 2013;42:5270. doi: 10.1039/c3cs35521g.
- 6.a) Quasdorf KW, Antoft-Finch A, Liu P, Silberstein AL, Komaromi A, Blackburn T, Ramgren SD, Houk KN, Snieckus V, Garg NK. J Am. Chem. Soc. 2011;133:6352. doi: 10.1021/ja200398c. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Ramgren SD, Hie L, Ye Y, Garg NK. Org. Lett. 2013;15:3950. doi: 10.1021/ol401727y. [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Huang K, Li G, Huang W-P, Yu D-G, Shi Z-J. Chem. Commun. 2011;47:7224. doi: 10.1039/c1cc11193k. [DOI] [PubMed] [Google Scholar]
- 7.a) Percec V, Golding GM, Smidrkal J, Weichold O. J Org. Chem. 2004;69:3447. doi: 10.1021/jo049940i. [DOI] [PubMed] [Google Scholar]; b) Zhao Y-L, Li Y, Li S-M, Zhou Y-G, Sun F-Y, Gao L-X, Han F-S. Adv. Synth. Catal. 2011;353:1543. [Google Scholar]
- 8.a) Huang K, Li G, Huang W-P, Yu D-G, Shi Z-J. Chem. Commun. 2011;47:7224. doi: 10.1039/c1cc11193k. [DOI] [PubMed] [Google Scholar]; b) Yu D-G, Shi Z-J. Angew. Chem., Int. Ed. 2011;50:7097. doi: 10.1002/anie.201101461. [DOI] [PubMed] [Google Scholar]; c) Tobisu M, Xu T, Shimasaki T, Chatani N. J Am. Chem. Soc. 2011;133:19505. doi: 10.1021/ja207759e. [DOI] [PubMed] [Google Scholar]
- 9.Jana R, Pathak TP, Sigman MS. Chem. Rev. 2011;111:1417. doi: 10.1021/cr100327p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sera M, Yamashita M, Ono Y, Tabata T, Muto E, Ouchi T, Tawada H. Org. Process Res. Dev. 2014;18:446. [Google Scholar]
- 11.Galland J-C, Savignae M, Genet J-P. Tetrahedron Lett. 1999;40:2323. [Google Scholar]
- 12.Ge S, Hartwig JF. Angew. Chem., Int. Ed. 2012;51:12837. doi: 10.1002/anie.201207428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Park NH, Teverovskiy G, Buchwald SL. Org. Lett. 2014;16:220. doi: 10.1021/ol403209k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ramgren SD, Hie L, Ye Y, Garg NK. Org. Lett. 2013;15:3950. doi: 10.1021/ol401727y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sheldon RA. Green Chem. 2007;9:1273. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.