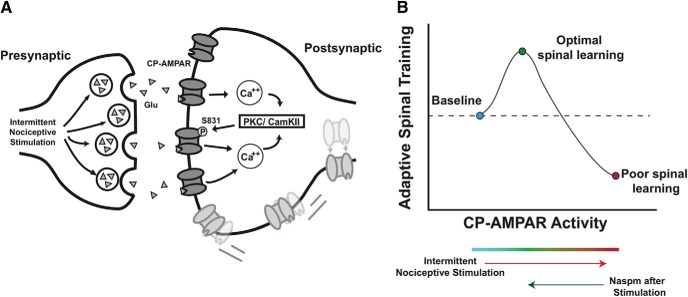
Figure 7.
A, Theoretical pathway underlying INS-induced maladaptive plasticity. Following afferent intermittent nociceptive stimulation, increased glutamate release engages postsynaptic AMPA receptors. Calcium influx via CP-AMPARs activates the calcium detectors PKC and/or CamKI phosphorylating the serine 831 site on GluA1 AMPAR subunit. Serine 831 phosphorylation increases the open probability of AMPARs, creating a feedforward loop that leads to membrane insertion of extrasynaptic CP-AMPARs. These receptors are trafficked laterally to the synaptic membrane, further strengthening this excitatory connection. B, Conceptual model of CP-AMPAR effects on spinal plasticity after SCI. CP-AMPAR activity critically shapes synaptic strength and use-dependent spinal cord plasticity after injury. Peripheral stimulation below the injury engages CP-AMPAR-mediated calcium influx, activating intracellular modulators of synaptic plasticity and strengthening excitatory tone to promote adaptive spinal training. However, CP-AMPARs are hyper-responsive to peripheral input (eg, limb positioning; skin stimulation) and are easily overdriven, resulting in synaptic saturation that overwhelms the capacity for adaptive spinal cord learning. As CP-AMPAR activity further increased, excitotoxicity and cell death may occur. Therapeutic intervention to decrease CP-AMPAR over-activity normalizes the balance of synaptic GluA1 and GluA2, and restores optimal adaptive plasticity.