Abstract
Objective
We aimed to identify biomarkers of Alzheimer's disease (AD) in order to improve diagnostic accuracy at mild stage.
Methods
AD patients aged >50 years were included in the disease group. We evaluated the relationship between potential blood and cerebrospinal fluid inflammatory biomarkers, cognitive status, temporal lobe atrophy and disease severity. Inflammatory biomarkers including interleukin 6 (IL-6), IL-18, fractalkine and tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) levels were measured. APOE genotypes were determined.
Results
We enrolled 41 subjects in the disease group and 40 subjects in the normal control group. The majority (88.9%) of subjects in the disease group had mild AD. Elevated levels of plasma IL-6 and decreased levels of plasma TRAIL in the disease group were noted. Plasma levels of IL-6 and TRAIL were significantly correlated with their cerebrospinal fluid levels.
Conclusion
Plasma IL-6 and TRAIL were identified as potential biomarkers of AD at an early stage.
Key Words: Alzheimer's disease, Biomarker, IL-6, TRAIL, Neuroinflammation
Introduction
Alzheimer's Disease and Biomarkers
Alzheimer's disease (AD) dementia refers to the clinical syndrome that arises as a consequence of the AD pathophysiological process, while results in the loss of neurons from widespread areas of the brain. Accumulation of β-amyloid (Aβ) remained the hallmark in AD pathology and soluble oligomeric Aβ42 neurotoxicity has been believed to be the causative agent. The diagnosis of definite AD relies upon neuropathology of the brain. Nevertheless, robust diagnosis at an early stage is more important in clinical practice. Although the clinical diagnostic criteria for probable AD described by the National Institute of Neurological and Communicative Disorders and Stroke (NINCDS) and the Alzheimer's Disease and Related Disorders Association (ADRDA) have been applied over 25 years, their sensitivity was 81% specificity 70% after pathological confirmation [1,2]. Many challenges appeared especially in the early diagnosis of AD.
Identification of biomarkers is an important step to improve the accuracy of early AD diagnosis. Applications of neuroimaging including magnetic resonance imaging (MRI), positron emission tomography with [18F] fluorodeoxyglucose (FDG-PET), and amyloid imaging might help in raising sensitivity or specificity in the diagnosis of AD. However, in daily practice the use of neuroimaging is impeded by cost and availability. Furthermore, incorporation of cerebrospinal fluid (CSF) biomarkers provides more diagnostic/prognostic information. Abnormally low Aβ and/or high tau levels can help predict pathological changes associated with AD. However, two- to three-fold differences in the cutoff values of total tau and Aβ levels were found for the same kit across different studies [3]. In addition, the procedure of spinal tapping is more invasive than venipuncture and is not easily accepted by patients suspected to have AD. Blood samples are much easier to obtain than CSF, and the cost of venipuncture is far less than that of neuroimaging. Until now, numerous potential biomarkers for AD in peripheral blood have been reported, but large discrepancies exist among different studies. A consensus on a standard protocol for blood sample collection and storage is lacking.
Inflammatory Cytokines
Neuroinflammation is commonly seen in the postmortem brains of AD patients. Increased oxidative stress markers were found in brains of amnestic mild cognitive impairment subjects, most of whom with pre-AD [4]. Chronic inflammation was proposed as a dysregulated mechanism in AD patients [5]. Aβ has been shown to induce expression of interleukin 6 (IL-6) in astrocytes and microglia in culture [6]. In hippocampal neurons, Aβ and IL-6 were both able to induce synaptic dysfunction [7]. Several studies attempted to prove the validity of IL-6 levels in the serum or CSF as a biomarker for AD. Nevertheless the results were inconclusive [8,9]. Increased blood levels of IL-18 were reported in AD patients [10,11]. IL-18 has a direct neuromodulatory role in synaptic plasticity and is involved in numerous inflammatory processes [12]. The chemokine fractalkine (CX3CL1) mediates neuron-microglia communication in neurodegenerative diseases, including tauopathies, another hallmark found in AD pathology [13]. Increased levels of fractalkine were found in the hippocampus tau-injured neurons of AD patients [14] and the plasma levels of AD patients and subjects with mild cognitive impairment [15]. Tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) has a dual role in inflammation. The TRAIL receptor could mediate oligomeric Aβ-induced apoptosis [16]. An inverse correlation between serum TRAIL levels and Mini-Mental State Examination (MMSE) has been discussed in a group with a small number of AD patients [17].
Analysis of Potential Biomarkers both in Plasma and CSF
Few reports measured biomarkers both in plasma and CSF of AD patients [18]. Until now, the mechanism of how cytokines in the central nervous system contribute peripheral cytokine is still unknown. Whether plasma Aβ could reflect CSF Aβ or Aβ in the brain is also inconclusive. It has been reported that plasma and CSF Aβ concentrations were not related on an individual basis [19].
Neuroimaging Biomarker
The mesial temporal lobe, especially the hippocampus, has emerged as the most sensitive area to be examines for AD-related atrophy. The radial width of the temporal horn (rWTH) on computed tomography (CT) scans is sensitive to the regional brain atrophy common in early AD [20,21] and pathologically validated [22]. Compared to hippocampal volumetry, CT measurement of rWTH was much less time-consuming and had less error in operation. Hippocampal volumetry required individual segmentation of structures within the mesial temporal lobe, which may vary across different operators [23]. The measurement of rWTH may provide clinically value for early diagnosis of AD.
Objective of This Study
Our aims were to identify clinically useful plasma biomarkers and to find the relationship between their peripheral and central distribution. We also wanted to examine the correlation between neuroimaging and neurocognitive tests.
Materials and Methods
Study Design
From October 2010 to September 2012 subjects were recruited at Shin-Kong WHS Memorial Hospital with institutional review board approval (20100806R). Informed consent was obtained from participants and/or their primary caregivers. We enrolled patients with AD into the AD group. The diagnosis of AD fulfilled the revised NINCDS criteria of the year 2007 [24]. Any subjects who had significant cerebrovascular disease revealed by brain CT or MRI were excluded. Subjects enrolled in the normal control group complained of no memory problem. For both groups, all participants were aged >50 years and had no major psychiatric disorders, history of head trauma or clinically relevant uncontrolled hypertensive, cardiopulmonary, metabolic or hematological diseases.
Neuropsychological Assessments
Cognitive function tests were performed by experienced clinical psychologists. For subjects in the AD group, the Neuropsychology Battery developed by the Consortium to Establish a Registry for Alzheimer's Disease (CERAD) was applied. CERAD included tests of verbal fluency (naming animals), a modified 15-item Boston Naming Test, MMSE, word list memory, recall, and recognition, constructional praxis and recall of constructional praxis. Differences between MMSE score with age and education-adjusted reference score were defined as rMMSE. Disease severity was estimated by Clinical Dementia Rating (CDR). In subjects diagnosed as AD, a CDR of 0.5 or 1 indicates mild stage, where a CDR of 2 indicates moderate stage and a CDR ≥3 indicates severe stage. Subjects in the normal control group had to have an AD8 score <2 or a Montreal Cognitive Assessment (MoCA) score >26 [25].
Calculation of the Temporal Atrophy Index
Estimates of temporal lobe atrophy were computed from CT images, which were obtained by a single Siemens sensation 16 (16-slice) CT scanner. On CT films, the scan where the temporal horn could be appreciated in its full length was chosen. Two parallel lines were drawn tangential to the tip of the horn where the width was largest. The rWTH was the distance between the two lines. The widths of the most anterior aspect of the temporal horn were measured on both sides at the level of the circle of Willis. The corresponding width between the bilateral inner table of the skull bone was measured and defined as skull width. The temporal atrophy index was defined as (right temporal horn width + left temporal horn width)/skull width × 100%. All measurements and calculations were done by an experienced investigator using the Picture Archiving and Communication System (PACS).
Spinal Tapping Procedure
Subjects who agreed to undergo spinal tapping received the procedure in the lateral decubitus position. After local anesthesia a spinal needle was inserted into the level of the L4-5 interspace. The collected CSF was sent to the laboratory for analysis of cell count, protein, glucose, Gram stain and bacterial culture. Another 3 ml of the collected CSF was stored at −80°C within 1 h for further analysis.
Blood Sample Preparation
For each subject, a 12-16 ml blood sample was drawn via venipuncture. No fasting was required. The drawn blood was aliquoted into two BD Vacutainer® Blood Collection Tubes containing K2 EDTA and stored immediately at 4°C. Within 3 h of blood sampling, centrifugation at 3,000 rpm for 10 min at 4°C was done. The supernatants were aliquoted into polypropylene tubes and protease inhibitors (Roche, Germany) were added. The prepared plasma samples were stored at −80°C until ELISA assays.
ELISA
Plasma samples were analyzed for IL-6, IL-18, fractalkine and TRAIL by following specific commercial plate-based ELISAs: (1) Human IL-6 Quantikine Kit (R&D Systems, USA), (2) Human IL-18 Kit (IBL, Japan), (3) Human Fractalkine Quantikine Kit (R&D Systems, USA), and (4) Human TRAIL Quantikine Kit (R&D Systems, USA). Assays were performed according to the manufacturer's instructions.
Apolipoprotein E Genotyping
Genomic DNA was extracted using Puregene® Blood Core Kit B (QIAGEN®, USA). Specific primers and probes designed by Applied Biosystems Inc. (USA) were used to detect two single nucleotide polymorphisms that could classify allelic variants (ε2, ε3, ε4) of the APOE gene. Assays were operated according to the manufacturer's instructions. The primers distinguish the ε2 allele from the ε3 and ε4 alleles at amino acid position 158 (NCBI rs7412) and the ε4 allele from the ε2 and ε3 alleles at amino acid position 112 (NCBI rs429358). TaqMan real-time polymerase chain reaction (RT-PCR) assays were performed in an ABI 7900 HT RT-PCR system using a cycling program of 95°C for 10 min, 40 cycles of 95°C for 15 s, and 60°C for 1 min. One negative control (water) was included in each plate.
Statistical Analyses
All statistical analyses were performed using the GraphPad Prism version 5.0 statistical software. χ2 tests were used to compare gender data in the two groups. The Kolmogorov-Smirnov test was used for normality test for plasma protein levels in the normal control group, data set of cognitive tests, and temporal lobe atrophy. The quantitative data were expressed as mean and standard deviation. We used unpaired t tests to compare levels of biomarkers between the two groups with equal variances. Unequal variance t tests (with Welch's correction) were applied to compare levels of biomarkers between the two groups with unequal variances. Pearson correlation analysis was used for the relation between plasma and CSF biomarker levels. Correlation analyses between biomarker level and age were done for an identified biomarker, with Pearson tests used for TRAIL and Spearman tests used for IL-6. Age-adjusted linear regression was used for correlation analysis of the CERAD score and the temporal atrophy index. The regression formula was presented as Yˆ = A + B1(X1) + B2(X2), with ‘Yˆ’ referring to the expected levels of biomarker, ‘A’ referring to constant, ‘B1’ referring to regression coefficient of X1 (age) and ‘X2’ referring to group (dummy variable). The regression coefficient B2 associated with the dummy variable was interpreted as the expected difference in the mean of the outcome variable for that AD group as compared to the normal control group, holding all other predictors constant. All test results are presented as two-tailed p values, and p < 0.05 was considered statistically significant.
Results
Demographic Data
We included a total of 41 subjects with AD as the AD group and 40 subjects without memory problems as the normal control group (table 1). The male:female ratio was similar between the two groups. Mean age was higher and mean education was lower in the AD group. Six subjects (14.6%) were under acetylcholinesterase inhibitor treatment when the blood sample was obtained and none was under memantine. The numbers of ApoE4 carriers were 9 (22.0%) in the AD group and 4 (10.0%) in the normal control group. All ApoE4 carriers were heterozygous. Allele frequencies of the APOE gene in the AD group were highest for ε3 (92.5%), 11.25% for ε4, and lowest for ε2 (2.5%). Allele frequencies of the APOE gene in the normal control group were highest for ε3 (86.25%), 7.5% for ε2, and lowest for ε4 (6.4%).
Table 1.
Demographic data of the AD group and the normal control group
| AD (n = 41) | Control (n = 40) | χ2 or t or U test | |
| Age, years | 73.1 ±9.4 | 63.0± 5.6 | p < 0.0001a |
| Education, years | 5.4 ±3.8 | 10.6± 4.2 | p < 0.0001b |
| Gender, M/F | 14/27 | 13/27 | n.s.c |
| ApoE4 allele frequency | 11.25%d | 6.4% |
Data are presented as mean ± standard deviation unless indicated otherwise. Mean age was higher and mean education lower in the AD group. No gender difference was found between the two groups.
Unequal variance t test (with Welch's correction).
Mann-Whitney U test.
Comparison between gender distribution of the two groups was done by χ2 test (not significant).
n = 40, ApoE genotyping not available for one subject in the AD group.
Scores of Global Cognitive Status
Cognitive tests were only performed in the AD group. The mean MMSE in the AD group was 17.5 (table 2). The ratio of mild AD (CDR = 0.5 or 1) was 87.8% (table 3).
Table 2.
Cognitive test data in the AD group
| Test (full score) | Mean | Range |
|---|---|---|
| MMSE | 17.5 | 8 to 27 |
| rMMSE | −1.7 | −14 to 7 |
| Clock drawing | 5.4 | 0 to 10 |
| CERAD | 48.8 | 18 to 76 |
| CDR | 0.86 | 0.5 to 4 |
Table 3.
Prevalence of CDR score in the AD group
| CDR | 0.5 | 1 | ≥2 |
|---|---|---|---|
| Number (%) | 23 (56.1) | 13 (31.7) | 5 (12.2) |
Correlations of Neurocognitive Scores and Temporal Atrophy Index
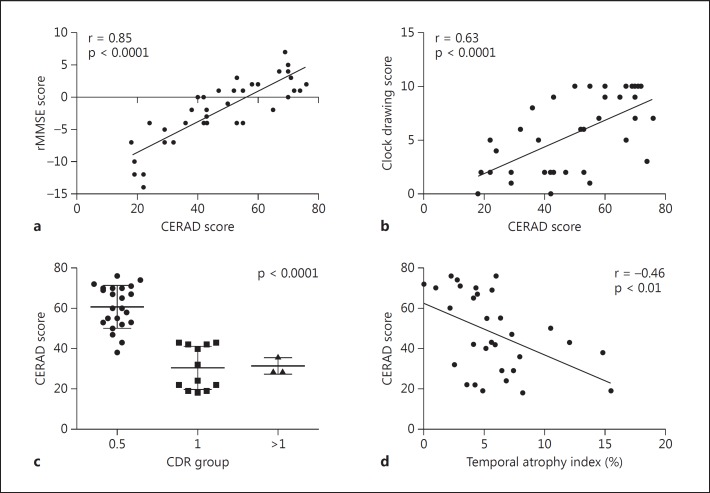
The CERAD score and the MMSE passed Kolmogorov-Smirnov normality tests. CERAD was highly correlated with rMMSE and clock drawing (r = 0.85 and r = 0.63, respectively, p < 0.0001) (fig. 1a, b). A significantly reduced CERAD score as CDR increased was noted by Kruskal-Wallis test (p < 0.0001) (fig. 1c). A negative correlation between CERAD and temporal atrophy index was noted (r = −0.46, p < 0.01, age-adjusted linear regression p < 0.001) (fig. 1d). The clock drawing score also showed a negative correlation with the temporal atrophy index (r = −0.37, p < 0.05).
Fig. 1.
Relationships between cognitive test scores and temporal atrophy index. a, b Positive correlation between CERAD score and rMMSE (a) and between CERAD score and clock drawing test score (b), analyzed by Pearson correlation (both p < 0.0001). c Reduced CERAD score as disease severity increased, analyzed by Kruskal-Wallis test (p < 0 0001). d Negative correlation between temporal atrophy index and CERAD score, analyzed by Pearson correlation (p < 0.01).
Concentrations of Potential Plasma Biomarkers
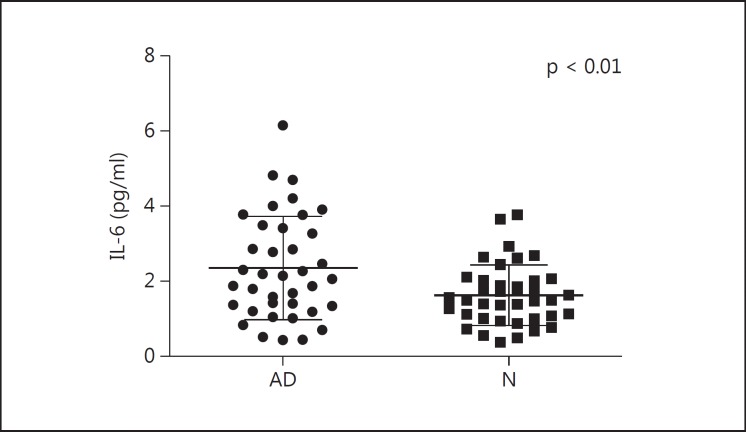
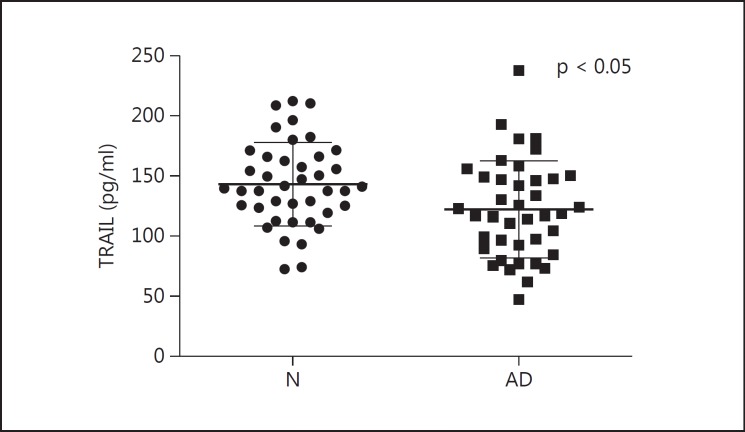
We compared plasma levels of pathogenic proteins and cytokines between the AD group and the normal control group (table 4). Plasma levels of IL-6, fractalkine and TRAIL in the control group were normally distributed by Kolmogorov-Smirnov test. Data of plasma levels of IL-6 were transformed by log normalization for further analyses (fig. 2). Plasma levels of TRAIL decreased in the AD group (p < 0.05) (fig. 3). Age did not correlate with plasma levels of IL-6 or TRAIL. No significant difference between the two groups was noted in the plasma levels of IL-18 and fractalkine.
Table 4.
Comparison of plasma biomarkers in the AD and in the normal control group
| AD | Control | t or U test | |
|---|---|---|---|
| IL-6, pg/ml | 2.343 ± 1.379 (n = 38) | 1.622± 0.806 (n = 39) | p = 0.0071a* |
| IL-18, pg/ml | 250.6 ± 141.8 (n = 38) | 249.6± 132.0 (n = 39) | p = 0.6782 |
| Fractalkine, pg/ml | 618.2 ± 209.5 (n = 39) | 580.6± 114.3 (n = 36) | p = 0.3339a |
| TRAIL, pg/ml | 121.8 ±40.42 (n = 41) | 143.0± 34.56 (n = 40) | p = 0.0132* |
Data are presented as mean ± standard deviation. Plasma levels of IL-6 were elevated in the AD group compared to the normal control group. No group difference in plasma levels of IL-18 or fractalkine was noted. Decreased levels of plasma TRAIL were found in the AD group.
Plasma biomarkers were compared using unequal variance (Welch) t tests.
p < 0.05.
Fig. 2.
Plasma levels of IL-6 in the AD and in the normal control group (N). Elevated plasma levels of IL-6 in the AD group compared to the normal control group by unequal variance t test (with Welch's correction) are seen.
Fig. 3.
Plasma levels of TRAIL in the AD and in the normal control group (N). Decreased plasma levels of TRAIL in the AD group compared to the normal control group by unpaired t test are seen.
Correlation of Neurocognitive Scores and Concentrations of Potential Plasma Biomarkers
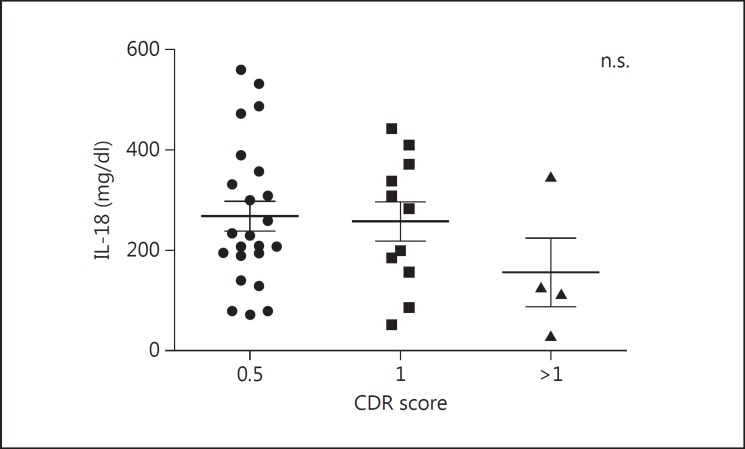
Furthermore, we determined whether the level of these biomarkers could reflect disease severity. Although no significant correlation was found, plasma IL-18 showed a declining trend as disease progressed (fig. 4).
Fig. 4.
Levels of IL-18 in different disease severities. No significant difference among plasma IL-18 levels of different disease severities was found by Kruskal-Wallis test, although the mean level in the CDR >1 group was reduced compared to the CDR = 0.5 or the CDR = 1 group. n.s. = Not significant.
Correlation of Plasma and CSF Concentrations of Potential Biomarkers
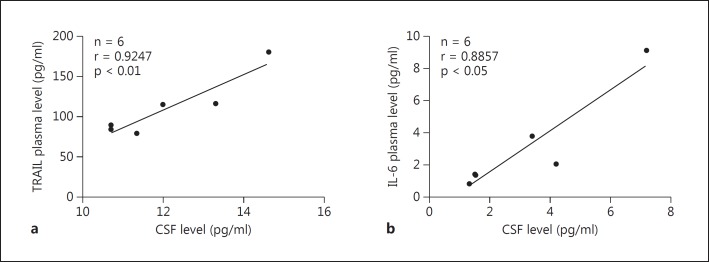
In addition, we examined whether the levels of cytokines in plasma could reflect their levels in CSF. In a small number of subjects, plasma levels of TRAIL and IL-6 were highly correlated with their CSF levels (p < 0.01 and p < 0.05, respectively) (fig. 5).
Fig. 5.
Correlation between CSF and plasma levels of TRAIL (a) and IL-6 (b). Highly correlated plasma levels of TRAIL and IL-6 with each of their CSF levels were found by Pearson correlation.
Discussion
Elevate Inflammatory Cytokine IL-6 and Reduce TRAIL in Early AD
This study disclosed the changes of neuroinflammatory proteins in early AD. AD patients have higher levels of plasma IL-6 and lower levels of TRAIL than controls. Levels of IL-6 and TRAIL in plasma and CSF were significantly correlated with each other. In addition, we report a negative correlation between CERAD and temporal atrophy index. Senile plaques in AD brains are associated with reactive astrocytes and activated microglial cells, expressing a number of inflammatory cytokines such as IL-6 [26]. The neuroimmunological cascade has been assumed as a major contribution to neuronal dysfunction. Interrelationship among cholinergic neurotransmission, soluble Aβ and Aβ-induced production of proinflammatory cytokines contributes to AD pathology [27]. In our study, we found that plasma levels of IL-6 were elevated in the AD group, which is consistent with a result from a large-scale meta-analysis [11]. IL-6 may play multiple roles in aging and neurodegeneration. Elderly without dementia who had higher IL-6 levels in blood had reduced hippocampal volumes [28]. The level of serum IL-6 was also found to be elevated in subjects with metabolic syndrome [29], which might play a role in the onset of AD [30]. The elevation of plasma IL-6 probably started in the very early stage of AD.
Until now, only one study has reported that plasma levels of TRAIL were decreased in AD [17]. We confirmed that the plasma TRAIL level was decreased in the AD group with a large number of subjects, but this level did not correlate with age. This proapoptotic tumor necrosis factor family cytokine has been largely studied as a cancer therapeutic. TRAIL plays broader roles in regulating immune processes, suggested by its widespread expression under inflammatory conditions and its ability to induce both apoptotic and pro-survival signaling pathways [31]. TRAIL could activate caspases to cleave Beclin-1 and Atg5, thus leading to cytotoxicity [32]. Neutralization of TRAIL can protect Aβ-induced toxicity in vitro [33]. Plasma TRAIL emerges as another candidate biomarker for early AD and TRAIL-targeting therapeutics may have potential new applications.
Highly Correlated Plasma and CSF Inflammatory Cytokines (IL-6 and TRAIL)
Highly correlated plasma and CSF biomarker levels found in IL-6 and TRAIL might implicate a strong connection between peripheral and central inflammatory cytokines. Many hypotheses were proposed for interchange between peripheral and central inflammatory cytokines [34,35]. A reverse correlation between the level of CSF IL-6 and anxiety was reported, without data of plasma levels [36]. As we found elevation of plasma IL-6 and reduction of plasma TRAIL in early AD, plasma levels of these two cytokines might reflect proportional changes of those in the central nervous system. This trend might be consistent with evidence of CSF IL-6 elevation in the early stage of AD [37,38].
No Significant Difference in IL-18 and Fractalkine
IL-18 is a regulator of both cellular and humoral immunity and has both physiological and pathological roles in the central nervous system. IL-18 may act directly on the neurons of the hippocampus, cerebral cortex and cerebellum [39]. Together with IL-12, IL-18 can stimulate the production of gamma interferon in Th1 cells and Th2 cytokines. Evidence for elevated and unchanged serum level of IL-18 has been reported [40]. IL-18 overexpression has been shown to initiate inflammatory processes in the brains of AD patients [41]. However, we did not detect significant difference in plasma IL-18 or fractalkine between the two groups. Only a declining trend in plasma IL-18 as disease progressed was found.
Significance of Application of Neuroimaging and Neurocognitive Tests on AD Patients
In this study, the temporal atrophy index in CT imaging was negatively correlated with the cognitive test CERAD score, especially clock drawing. In other words, AD patients with a higher rate of temporal atrophy showed worse cognitive function. This correlation has not been mentioned before and strengthens the value on clinical diagnosis. Moreover, the temporal atrophy index is a simple way of estimating temporal atrophy. Clock drawing is a component of CERAD and MoCA, but not of MMSE. The test involves frontal battery and is helpful in determining subjects with a potential risk of cognitive impairment [42,43]. Semantic impairments play a major role in AD patients' poor clock drawing. AD patients easily make stimulus-bound errors, such as placing the clock hands on the number 10 and 11 or writing out the time using letters or numbers. Assessment of clock drawing-related errors for making an AD diagnosis might be more helpful than memory tests, which often demonstrate a floor effect, even in the very early stage of AD [44]. Clinical application of the clock drawing test remains crucial both in screening and in the evaluation of cognitive decline.
Study Limitations
The limitations of this study might have unknown influences on the results and include (1) a relative larger proportion of subjects with younger age and higher education level in the normal control group, (2) cholinesterase inhibitor use among some AD patients, (3) lack of information on body mass index or nutrition intake and (4) no control of individual variation in blood-CSF barrier integrity. Although our findings may depend in part on the analysis algorithms used, they suggest plasma IL-6 and TRAIL as potential biomarkers of AD, especially in differentiating early AD subjects from the normal population. Plasma IL-6 and TRAIL could reflect the changes of cytokines in the brain, which needs further large-scale analysis. We hope that our results will contribute to the early diagnosis of AD.
Disclosure Statement
The authors declare that there is no conflict of interest.
Acknowledgements
This study was supported by grants from Shin-Kong WHS Memorial Hospital, Taipei, Taiwan (No. SKH 8302-101-20100806R) and partly by grants from the Taiwan Ministry of Science and Technology (NSC 102-2320-B-010-021-MY2), the National Health Research Institute (NHRI-EX103-10338NI) and Cheng Hsin General Hospital (102F218C05).
References
- 1.Knopman DS, et al. Practice parameter: diagnosis of dementia (an evidence-based review). Report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology. 2001;56:1143–1153. doi: 10.1212/wnl.56.9.1143. [DOI] [PubMed] [Google Scholar]
- 2.Beach TG, Monsell SE, Phillips LE, Kukull W. Accuracy of the clinical diagnosis of Alzheimer disease at National Institute on Aging Alzheimer Disease Centers, 2005-2010. J Neuropathol Exp Neurol. 2012;71:266–273. doi: 10.1097/NEN.0b013e31824b211b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hort J, Bartos A, Pirttila T, Scheltens P. Use of cerebrospinal fluid biomarkers in diagnosis of dementia across Europe. Eur J Neurol. 2010;17:90–96. doi: 10.1111/j.1468-1331.2009.02753.x. [DOI] [PubMed] [Google Scholar]
- 4.Keller JN, et al. Evidence of increased oxidative damage in subjects with mild cognitive impairment. Neurology. 2005;64:1152–1156. doi: 10.1212/01.WNL.0000156156.13641.BA. [DOI] [PubMed] [Google Scholar]
- 5.Krstic D, Knuesel I. Deciphering the mechanism underlying late-onset Alzheimer disease. Nat Rev Neurol. 2012;9:25–34. doi: 10.1038/nrneurol.2012.236. [DOI] [PubMed] [Google Scholar]
- 6.Lee KS, et al. Peripheral cytokines and chemokines in Alzheimer's disease. Dement Geriatr Cogn Disord. 2009;28:281–287. doi: 10.1159/000245156. [DOI] [PubMed] [Google Scholar]
- 7.Walsh KP, et al. Amyloid-beta and proinflammatory cytokines utilize a prion protein-dependent pathway to activate NADPH oxidase and induce cofilin-actin rods in hippocampal neurons. PLoS One. 2014;9:e95995. doi: 10.1371/journal.pone.0095995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Anoop A, Singh PK, Jacob RS, Maji SK. CSF biomarkers for Alzheimer's disease diagnosis. Int J Alzheimers Dis. 2010;2010:606802. doi: 10.4061/2010/606802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Spooren A, et al. Interleukin-6, a mental cytokine. Brain Res Rev. 2011;67:157–183. doi: 10.1016/j.brainresrev.2011.01.002. [DOI] [PubMed] [Google Scholar]
- 10.Motta M, Imbesi R, Di Rosa M, Stivala F, Malaguarnera L. Altered plasma cytokine levels in Alzheimer's disease: correlation with the disease progression. Immunol Lett. 2007;114:46–51. doi: 10.1016/j.imlet.2007.09.002. [DOI] [PubMed] [Google Scholar]
- 11.Swardfager W, et al. A meta-analysis of cytokines in Alzheimer's disease. Biol Psychiatry. 2010;68:930–941. doi: 10.1016/j.biopsych.2010.06.012. [DOI] [PubMed] [Google Scholar]
- 12.Alboni S, Cervia D, Sugama S, Conti B. Interleukin 18 in the CNS. J Neuroinflammation. 2010;7:9. doi: 10.1186/1742-2094-7-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Desforges NM, Hebron ML, Algarzae NK, Lonskaya I, Moussa CE. Fractalkine mediates communication between pathogenic proteins and microglia: implications of anti-inflammatory treatments in different stages of neurodegenerative diseases. Int J Alzheimers Dis. 2012;2012:345472. doi: 10.1155/2012/345472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lastres-Becker I, et al. Fractalkine activates NRF2/NFE2L2 and heme oxygenase 1 to restrain tauopathy-induced microgliosis. Brain. 2014;137:78–91. doi: 10.1093/brain/awt323. [DOI] [PubMed] [Google Scholar]
- 15.Kim TS, et al. Changes in the levels of plasma soluble fractalkine in patients with mild cognitive impairment and Alzheimer's disease. Neurosci Lett. 2008;436:196–200. doi: 10.1016/j.neulet.2008.03.019. [DOI] [PubMed] [Google Scholar]
- 16.Fossati S, Ghiso J, Rostagno A. TRAIL death receptors DR4 and DR5 mediate cerebral microvascular endothelial cell apoptosis induced by oligomeric Alzheimer's Aβ. Cell Death Dis. 2012;3:e321. doi: 10.1038/cddis.2012.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Genc S, et al. TNF-related apoptosis-inducing ligand level in Alzheimer's disease. Neurol Sci. 2009;30:263–267. doi: 10.1007/s10072-009-0047-5. [DOI] [PubMed] [Google Scholar]
- 18.Linkov F, et al. Multiplex analysis of cytokines as biomarkers that differentiate benign and malignant thyroid diseases. Proteomics Clin Appl. 2008;2:1575–1585. doi: 10.1002/prca.200780095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huang Y, et al. Beta-amyloid dynamics in human plasma. Arch Neurol. 2012;69:1591–1597. doi: 10.1001/archneurol.2012.18107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Frisoni GB, et al. Radial width of the temporal horn: a sensitive measure in Alzheimer disease. AJNR Am J Neuroradiol. 2002;23:35–47. [PMC free article] [PubMed] [Google Scholar]
- 21.Frisoni GB, Rossi R, Beltramello A. The radial width of the temporal horn in mild cognitive impairment. J Neuroimaging. 2002;12:351–354. doi: 10.1111/j.1552-6569.2002.tb00143.x. [DOI] [PubMed] [Google Scholar]
- 22.Rossi R, Joachim C, Smith AD, Frisoni GB. The CT-based radial width of the temporal horn: pathological validation in AD without cerebrovascular disease. Int J Geriatr Psychiatry. 2004;19:570–574. doi: 10.1002/gps.1132. [DOI] [PubMed] [Google Scholar]
- 23.Rathakrishnan BG, Doraiswamy PM, Petrella JR. Science to practice: translating automated brain MRI volumetry in Alzheimer's disease from research to routine diagnostic use in the work-up of dementia. Front Neurol. 2014;4:216. doi: 10.3389/fneur.2013.00216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dubois B, et al. Research criteria for the diagnosis of Alzheimer's disease: revising the NINCDS-ADRDA criteria. Lancet Neurol. 2007;6:734–746. doi: 10.1016/S1474-4422(07)70178-3. [DOI] [PubMed] [Google Scholar]
- 25.Nasreddine ZS, et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53:695–699. doi: 10.1111/j.1532-5415.2005.53221.x. [DOI] [PubMed] [Google Scholar]
- 26.Cacquevel M, Lebeurrier N, Cheenne S, Vivien D. Cytokines in neuroinflammation and Alzheimer's disease. Curr Drug Targets. 2004;5:529–534. doi: 10.2174/1389450043345308. [DOI] [PubMed] [Google Scholar]
- 27.Schliebs R. Basal forebrain cholinergic dysfunction in Alzheimer's disease – interrelationship with beta-amyloid, inflammation and neurotrophin signaling. Neurochem Res. 2005;30:895–908. doi: 10.1007/s11064-005-6962-9. [DOI] [PubMed] [Google Scholar]
- 28.Satizabal CL, Zhu YC, Mazoyer B, Dufouil C, Tzourio C. Circulating IL-6 and CRP are associated with MRI findings in the elderly: the 3C-Dijon Study. Neurology. 2012;78:720–727. doi: 10.1212/WNL.0b013e318248e50f. [DOI] [PubMed] [Google Scholar]
- 29.Weiss TW, Arnesen H, Seljeflot I. Components of the Interleukin-6 transsignalling system are associated with the metabolic syndrome, endothelial dysfunction and arterial stiffness. Metabolism. 2013;62:1008–1013. doi: 10.1016/j.metabol.2013.01.019. [DOI] [PubMed] [Google Scholar]
- 30.Rios JA, Cisternas P, Arrese M, Barja S, Inestrosa NC. Is Alzheimer's disease related to metabolic syndrome? A Wnt signaling conundrum. Prog Neurobiol. 2014;121:125–146. doi: 10.1016/j.pneurobio.2014.07.004. [DOI] [PubMed] [Google Scholar]
- 31.Benedict CA, Ware CF. TRAIL: not just for tumors anymore? J Exp Med. 2012;209:1903–1906. doi: 10.1084/jem.20122235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Salminen A, et al. Impaired autophagy and APP processing in Alzheimer's disease: the potential role of Beclin 1 interactome. Prog Neurobiol. 2013;106-107:33–54. doi: 10.1016/j.pneurobio.2013.06.002. [DOI] [PubMed] [Google Scholar]
- 33.Cantarella G, et al. Neutralization of TRAIL death pathway protects human neuronal cell line from beta-amyloid toxicity. Cell Death Differ. 2003;10:134–141. doi: 10.1038/sj.cdd.4401143. [DOI] [PubMed] [Google Scholar]
- 34.McGeer PL, McGeer EG. Inflammation, autotoxicity and Alzheimer disease. Neurobiol Aging. 2001;22:799–809. doi: 10.1016/s0197-4580(01)00289-5. [DOI] [PubMed] [Google Scholar]
- 35.Kim E, et al. Metabolomic signatures in peripheral blood associated with Alzheimer's disease amyloid-beta-induced neuroinflammation. J Alzheimers Dis. 2014;42:421–433. doi: 10.3233/JAD-132165. [DOI] [PubMed] [Google Scholar]
- 36.Holmgren S, et al. Neuropsychiatric symptoms in dementia – a role for neuroinflammation? Brain Res Bull. 2014;108:88–93. doi: 10.1016/j.brainresbull.2014.09.003. [DOI] [PubMed] [Google Scholar]
- 37.Schuitemaker A, et al. Inflammatory markers in AD and MCI patients with different biomarker profiles. Neurobiol Aging. 2009;30:1885–1889. doi: 10.1016/j.neurobiolaging.2008.01.014. [DOI] [PubMed] [Google Scholar]
- 38.Martinez M, Fernandez-Vivancos E, Frank A, De la Fuente M, Hernanz A. Increased cerebrospinal fluid fas (Apo-1) levels in Alzheimer's disease. Relationship with IL-6 concentrations. Brain Res. 2000;869:216–219. doi: 10.1016/s0006-8993(00)02363-5. [DOI] [PubMed] [Google Scholar]
- 39.Alboni S, et al. Mapping of the full length and the truncated interleukin-18 receptor alpha in the mouse brain. J Neuroimmunol. 2009;214:43–54. doi: 10.1016/j.jneuroim.2009.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lindberg C, et al. Soluble interleukin-1 receptor type II, IL-18 and caspase-1 in mild cognitive impairment and severe Alzheimer's disease. Neurochem Int. 2005;46:551–557. doi: 10.1016/j.neuint.2005.01.004. [DOI] [PubMed] [Google Scholar]
- 41.Liu L, Chan C. The role of inflammasome in Alzheimer's disease. Ageing Res Rev. 2014;15:6–15. doi: 10.1016/j.arr.2013.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shoyama M, et al. Brain activity during the Clock-Drawing Test: multichannel near-infrared spectroscopy study. Appl Neuropsychol. 2011;18:243–251. doi: 10.1080/09084282.2011.595450. [DOI] [PubMed] [Google Scholar]
- 43.Konagaya Y, Watanabe T, Konagaya M. Cognitive function screening of community-dwelling elderly people using the clock drawing test – quantitative and qualitative analyses. Nihon Ronen Igakkai Zasshi. 2012;49:483–490. doi: 10.3143/geriatrics.49.483. [DOI] [PubMed] [Google Scholar]
- 44.Lee JH, et al. Longitudinal changes in clock drawing test (CDT) performance according to dementia subtypes and severity. Arch Gerontol Geriatr. 2011;53:e179–e182. doi: 10.1016/j.archger.2010.08.010. [DOI] [PubMed] [Google Scholar]