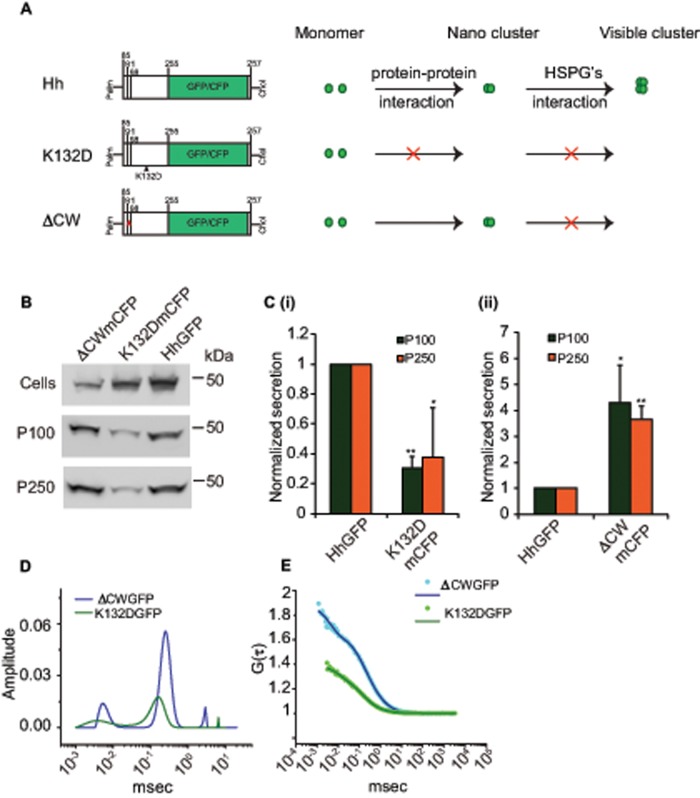
FIGURE 4:
Oligomerization of Hh is necessary for its vesicular release. (A) Cartoon representing different Hh variants with respective mutations used to gain mechanistic insights into the role of Hh organization in its secretion. Hh forms a nanoscale cluster on the cell surface through a protein–protein interaction, which is in turn essential for formation of optically resolvable clusters in association with HSPGs. A point mutation, K132D, results in disruption of an electrostatic interaction between Hh monomers that abolishes the hierarchical Hh clustering, whereas upon deletion of the HSPG-interacting CW (residues 91–98) domain it retains its ability to oligomerize at the nanoscale but cannot form optically resolvable clusters. (B, C) Western blots (B) reflect the amount of protein in the cellular (Cells) and secreted fractions (P100 and P250) of HhGFP, HhK132DmCFP, and HhΔCWmCFP expressed in S2R+ cells. (C) Bar graphs quantify the extent of secretion of HhK132DmCFP (i) and HhΔCWmCFP (ii) in both P100 and P250 fractions as compared with HhGFP. Nonsaturating exposures of cellular, P100, and P250 fractions were used for intensity measurements, and values in P100 and P250 fractions were normalized to the values in cell lysates. Data represent mean ± SD from three experiments; *p < 0.05, **p < 0.01. (D, E) Graphs showing distributions of diffusion time scales (D) of the different species present in the P100 fractions from S2R+ cells expressing HhK132DGFP (K132DGFP; green) and HhΔCWmCFP (ΔCWmCFP; blue) derived from the autocorrelation analysis of FCS data (E, dotted lines), using the MEM fitting routine as described in Figure 1 and used to compute the two-component, 3D diffusion fit of the FCS traces (solid lines in E).