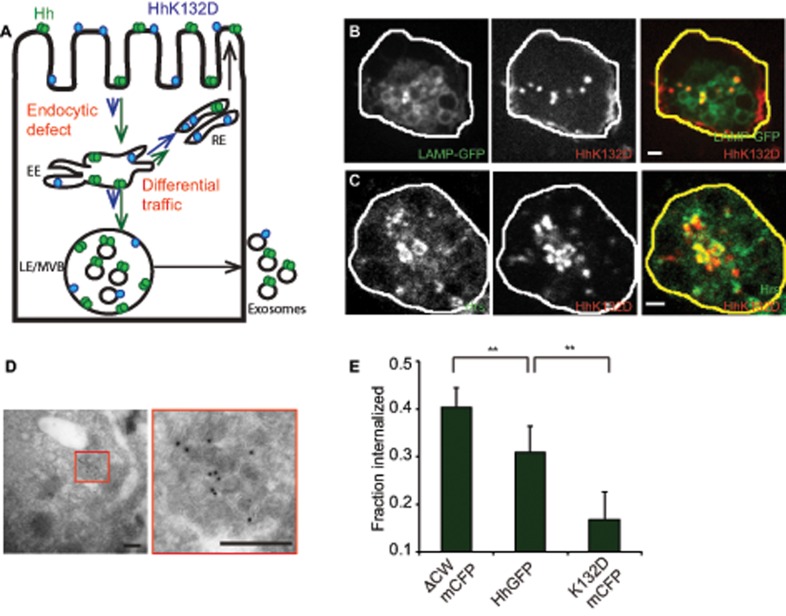
FIGURE 5:
Oligomerization-defective Hh is impaired in its endocytic delivery to the MVBs. (A) Model to explain the differences in trafficking of Hh variants to account for observed differences in secretion. Hh is represented in green as a homo-oligomer, and K132D is shown in blue in a monomeric form. The lengths of the arrows reflect the extent of traffic for each variant in a particular pathway. The extent of partitioning toward the recycling pathway of the Hh variants vs. the late endosomal pathway regulate delivery to the MVB and hence result in modulation of ILV formation. Alternatively, a more upstream endocytic block could also lead to similar consequences. (B, C) S2R+ cells expressing HhK132DmCFP were incubated with A647–anti-GFP Fab (red in merge) for 20 min (B) or 2 h (C) to localize internalized protein. It colocalizes with LAMP-GFP (B; green in merge) coexpressed in the same cells or with immunodetected Hrs (C; green in merge). Single confocal slices are shown from cells imaged using a PerkinElmer spinning-disk confocal microscope (B) or an Olympus FV1000 confocal microscope (C). Note that HhK132DmCFP traffics to LE/MVB structures and results in a redistribution of Hrs. Scale bar, 2 μm. (D) HhK132DmCFP is also visualized on ILVs inside the MVB lumen in cryosections of cells transfected with HhK132DmCFP labeled using anti-GFP antibody and detected using a 10-nm gold–conjugated secondary antibody. Scale bar, 200 nm. (E) Bar graphs showing the fraction of the surface-bound A647–anti-GFP Fab internalized in a 10-min endocytic pulse using a surface accessibility assay for each of the indicated Hh variants. Data from two experiments are represented as mean ± SEM from at least 50 cells for each variant; **p < 0.01.