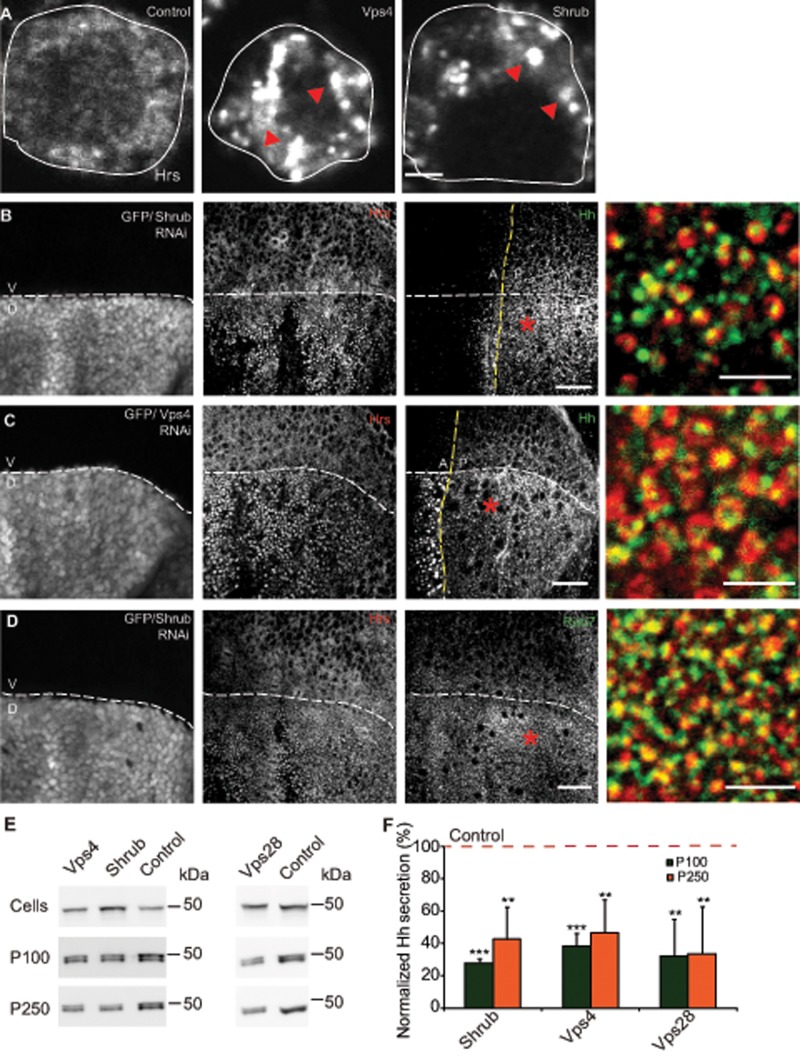
FIGURE 6:
Perturbations in ESCRT proteins result in intracellular Hh accumulation and affects its exovesicular secretion. (A) Endogenous Hrs (ESCRT 0; red arrowheads) immunostained using anti-Hrs antibody accumulates in vesicular structures in S2R+ cells upon Shrub (ESCRT3) and Vps4 RNAi treatments compared with treatment of cells using a control RNAi. Scale bar, 2 μm. (B, C) Drosophila wing imaginal discs from animals expressing Shrub RNAi (B) or Vps4 RNAi (C) under the control of Apterous-Gal4, marked by GFP fluorescence in the dorsal region, result in the local accumulation of Hh (green in merge) in large Hrs-positive (red in merge) compartments in the apical/subapical planes derived from a 3D stack of confocal images of the wing imaginal disc. Scale bar, 20 μm. Asterisk indicates regions from the posterior that are shown at a higher magnification. Scale bar, 5 μm. (D) Dorsal regions of Drosophila wing imaginal discs expressing Shrub RNAi driven using Apterous-Gal4 similar to B and marked by GFP fluorescence show that Hrs puncta (red in merge) stain for LE marker dRab7 (green in merge), confirming them as endocytic intermediates. Scale bar, 20 μm. Asterisk indicates a region that is shown at a higher magnification. Scale bar, 5 μm. (E) Western blots (top) reflect HhGFP in cellular (Cells) and secreted (P100 and P250) fractions (middle and bottom) derived from S2R+ cells transfected with HhGFP and grown in the presence of the indicated RNAi against ESCRT proteins and Vps4. (F) Bar graph showing the extent of reduction in normalized secretion (with respect to the control; red dashed line) of HhGFP in the P100 and P250 fractions in each of the indicated RNAi treatments. Nonsaturating exposures of cellular, P100, and P250 fractions were used for intensity measurements, and values in P100 and P250 fractions were normalized to the values in cell lysates. Data, expressed as mean ± SD, were derived from at least four independent experiments; *p < 0.05, **p < 0.01, ***p < 0.001.