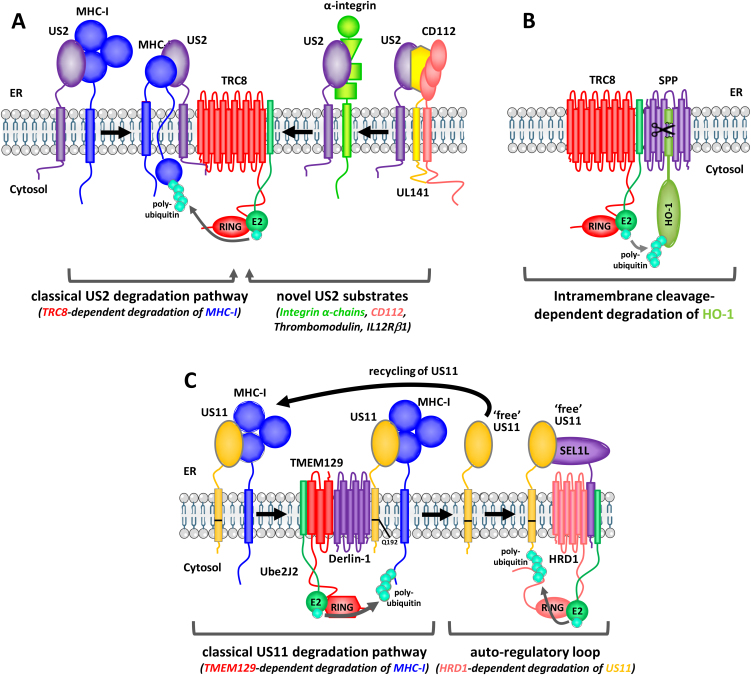
Fig. 1.
The US2 and US11 viral degradation hubs and their cognate host ERAD E3 ubiquitin ligases. (A) The US2/TRC8 degradation hub. US2 recruits the TRC8 E3 ligase via its cytosolic C-terminus. TRC8-dependent polyubiquitination of MHC-I leads to rapid retrotranslocation and degradation of the MHC-I heavy chain. In addition to MHC-I, the US2/TRC8 degradation hub degrades a wide variety of immune receptors including at least 6 different integrin α-chains, the anti-coagulation factor thrombomodulin, the IL-12 receptor β1 chain and the NK cell ligand CD112. Whereas US2/TRC8 is sufficient for the degradation of most substrates, efficient degradation of CD112 requires help from the HCMV-encoded ‘holdase’ UL141. US2 function is independent of SPP. (B) The TRC8/SPP degradation hub mediates cleavage dependent degradation of heme oxygenase-1 (HO-1). SPP-mediated cleavage of the HO-1 transmembrane domain releases HO-1 from the membrane and induces TRC8-dependent ubiquitination and proteasomal degradation. This pathway is used by other tail-anchored proteins. (C) The US11/TMEM129 degradation hub. US11-bound MHC-I is rapid ubiquitinated by the TMEM129 E3 ligase and subsequently retrotranslocated to the cytosol for proteasomal degradation. TMEM129 is recruited to US11 via Derlin-1. US11 is not itself a substrate for TMEM129, but is either recycled to bind another MHC-I molecule, or is degraded by the HRD1/SEL1L ERAD complex in an auto-regulation loop. In this way, HRD1-mediated degradation of ‘free US11’ buffers US11 levels to the MHC-I client load.