Summary
The classification of autoimmune hemolytic anemias and the complement system are reviewed. In autoimmune hemolytic anemia of the warm antibody type, complement-mediated cell lysis is clinically relevant in a proportion of the patients but is hardly essential for hemolysis in most patients. Cold antibody-mediated autoimmune hemolytic anemias (primary cold agglutinin disease, secondary cold agglutinin syndrome and paroxysmal cold hemoglobinuria) are entirely complement-mediated disorders. In cold agglutinin disease, efficient therapies have been developed in order to target the pathogenic B-cell clone, but complement modulation remains promising in some clinical situations. No established therapy exists for secondary cold agglutinin syndrome and paroxysmal cold hemoglobinuria, and the possibility of therapeutic complement inhibition is interesting. Currently, complement modulation is not clinically documented in any autoimmune hemolytic anemia. The most relevant candidate drugs and possible target levels of action are discussed.
Keywords: Complement, Autoimmune hemolytic anemia, Cold agglutinin disease, Therapy, Complement inhibitors
Introduction
Autoimmune hemolytic anemia (AIHA) is a collective term for several diseases characterized by autoantibody-initiated destruction of erythrocytes [1,2,3,4,5]. AIHA can be classified as shown in table 1. The insight into the etiology, pathogenesis, and therapy of these disorders is rapidly growing [3,4,5,6,7].
Table 1.
Autoimmune hemolytic anemia
| Warm-antibody type |
| Primary |
| Secondary |
| Cold-antibody type |
| Primary chronic cold agglutinin disease |
| Secondary cold agglutinin syndrome |
| Associated with malignant disease |
| Acute, infection-associated |
| Paroxysmal cold hemoglobinuria |
| Mixed cold- and warm-antibody type |
Our knowledge about the essential role of complement in subgroups of AIHA is also expanding [6,7,8], and possible therapeutic options for complement-modifying therapy are being investigated [9,10,11]. Moreover, although paroxysmal nocturnal hemoglobinuria (PNH) is not an autoimmune disorder, the entirely complement-dependent pathogenesis and the success of therapeutic complement inhibition in this disease makes it possible to learn lessons from PNH that might prove useful in treating AIHA [12,13].
This review will address the pathogenetic mechanisms of AIHA, focusing on the role of complement in RBC destruction and possible implications for the potential therapeutic use of complement modulators. Diagnostic procedures and established, non-complement targeted therapies will only be briefly mentioned.
The Complement System
The complement system is an essential part of the innate immune system. It is made up of numerous proteins that are widely distributed throughout body fluids and tissues [11,14]. Many of these plasma proteins are proteases that are themselves activated by proteolytic cleavage. At sites of infection and other cell surfaces, they can be activated locally and thereby trigger a cascade of enzymatic proteolysis and a series of potent inflammatory and lytic events. In such a cascade, cleavage of a precursor generates an active complement enzyme, which then cleaves its substrate, another complement protein, to its active enzymatic form. This in turn cleaves the next complement protein and thus activates successive enzymatic reactions. Figure 1 provides an overview of the complement system with focus on the steps relevant for AIHA [11,14,15].
Fig. 1.
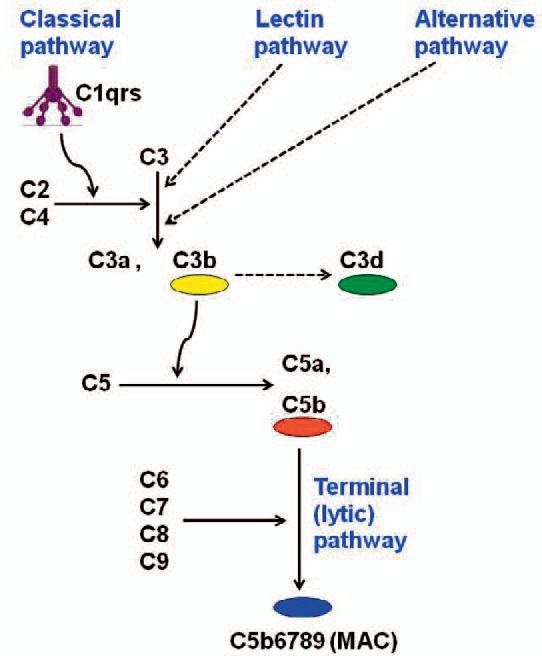
The complement cascade, simplified. Only parts relevant for this review article are shown. Explanation: See text. C = Complement; MAC = membrane attack complex. Originally published in BioMed Res Int 2015 [28]. Copyright: S. Berentsen and T. Sundic. Re-used with permission.
The classical complement pathway is initiated by binding of complement protein 1q (C1q), part of the C1 complex, to an antigen-antibody (AgAb) complex at a pathogen or host cell surface, allowing activation of C1r. Activated C1r cleaves C1s, generating an active serine protease that in turn cleaves C4 and C2. These reactions result in the formation of C3 convertase, which cleaves C3 into C3a, an anaphylotoxin, and C3b, which binds covalently to the pathogen or cell surface and acts as an opsonin as well as a further proteolytic enzyme [14,15,16,17].
Two other initial complement pathways are also known; the lectin pathway initiated by mannose-binding lectin and the alternative pathway triggered by the binding of spontaneous activated C3 in plasma to a pathogen surface. Like the classical pathway, these two reaction chains lead to the production of C3 convertase and, in turn, deposition of C3b on the cell surface. Thus, the formation of C3b is the point of convergence between the three initial complement pathways; and the classical pathway-initiating event of binding C1q represents a link between the adaptive immune system and the complement system [14,16,17]. The lectin and alternative pathways, believed to be less important for the pathogenesis in AIHA, will not be further addressed here.
Pathogens, or host cells in the case of autoimmunity, opsonized by C3b can bind to complement receptors on phagocytes. Such binding results in removal of C3b-opsonized cells by the reticulo-endothelial system, in particular in the spleen and/or liver. Concomitantly or alternatively, surface-bound C3b can be further degraded [3,14,16]. In the AIHA setting, such phagocytosis is known as extravascular hemolysis.
In addition to allowing phagocytic removal, surface-bound C3b can bind C3 convertase, forming C5 convertase, which initiates the terminal (lytic) complement pathway [14]. C5 convertase splits C5 into C5a, a powerful anaphylotoxin, and C5b, a surface-bound fragment that in turns binds C6, C7, C8 and C9. The C5b6789 complex, also known as the membrane attack complex (MAC), is capable of inducing cell lysis. In AIHA, therefore, activation of the terminal pathway will result in intravascular hemolysis.
Due to the amplifying nature of the cascades and positive feedback loops, activation of the complement system can lead to an accelerating, uncontrolled, and even fatal process of inflammatory reaction and cell lysis. Several physiologic complement inhibitors and negative feedback loops, however, prevent this from occurring under normal circumstances as well as in some pathologic conditions. Of relevance for complement-mediated hemolytic anemias, important cell-bound regulators are CD55, which has an inhibitory function at the C4-C2 level, and CD59, which prevents final assembly of the MAC at the C8-C9 stage [13,14].
Warm-Antibody Autoimmune Hemolytic Anemia
Etiology, Pathogenesis and Associated Disorders
The incidence of AIHA has been estimated to about 1:100,000 per year in adults [18] and even lower in children. Warm-antibody AIHA (w-AIHA) accounts for approximately 75% of the cases [1,2]. The autoantibodies in w-AIHA have temperature optimum at 37 °C and are invariably polyclonal, even when w-AIHA complicates a clonal B-cell lymphoproliferative disorder [19,20]. A general dysregulation of the immune system with impaired distinction between self and non-self seems essential to pathogenesis; and the T-cell-mediated regulation of the humoral immune system has been shown to play a critical role [20,21]. Polymorphism of the gene for the signal substance CTLA-4, which activates regulatory T cells (Treg cells), seems to bring about a disposition for autoimmunity [21].
It is not surprising, therefore, that a large number of immunological and lymphoproliferative disorders can be associated with w-AIHA. Secondary AIHA, i.e. cases with a demonstrable associated or underlying disease, accounts for about 50% of w-AIHA, while the remaining 50% are classified as primary. The most frequently occurring associated lymphoproliferative disease is chronic lymphatic leukemia (CLL), whereas w-AIHA complicating other non-Hodgkin's lymphomas (NHLs) is less common [1,2,19]. Furthermore, systemic lupus erythematosus, rheumatoid arthritis, Sjögren's syndrome, primary biliary cirrhosis, hypothyroidism, inflammatory bowel disease, immune thrombocytopenia as well as primary hypogammaglobulinemia and other immunologic diseases can be associated with w-AIHA [1,2,20,22]. Some patients have several associated diseases at the same time.
Autoantibody or complement fragment deposition on the RBC can usually be detected using polyspecific and monospecific direct antiglobulin test (DAT). The autoantibodies in w-AIHA are of the immunoglobulin G (IgG) class in most cases [4]. In up to 50% of w-AIHA, DAT is positive for complement fragments, most often C3d and usually in combination with IgG. IgA autoantibodies occur in 15-20% of the patients, either in combination with IgG or, less frequently, alone [23]. Cases with IgA as the sole autoantibody class may be misdiagnosed because reagents used in the polyspecific DAT do usually not contain anti-IgA. Warm autoantibodies of the IgM class have been assumed to be rare. Their frequency remains somewhat controversial, however, because they may have low affinity to the antigen and may have detached from the RBC surface before they can be detected by DAT [24,25]. In 3-10% of patients with w-AIHA, DAT is found to be negative [4,26]. The problem of ‘DAT-negative AIHA’ has been extensively discussed elsewhere in the literature [4,26,27,28].
Erythrocyte Destruction and Role of Complement in w-AIHA
Erythrocytes coated with warm-reactive autoantibodies are sequestered and phagocytosed by macrophages, primarily in the spleen [29,30,31]. The macrophage surface expresses receptors for the Fc region of the immunoglobulin molecules, which enables trapping and ingestion of the opsonized RBCs [32,33]. Often, however, phagocytosis is incomplete and results in formation of spherocytes [7,32]. This has been explained in part by the removal of more membrane than volume. Furthermore, ectoenzymes on the macrophage surface cause microperforations of the cell membrane, increasing its permeability and thereby promoting the transition from a biconcave to a spherical shape of the cell [7,29,31]. Spherocytes are prone to further destruction during subsequent passages through the spleen [4,7,30].
On erythrocytes heavily coated with immunoglobulin, the amount of AgAb complex may be sufficient for binding C1q and activation of the classical complement pathway (fig. 1) [15,34,35]. Unlike IgG, IgM is a potent complement activator but, as mentioned above, usually not found on the RBC surface by DAT in w-AIHA [24]. Regarding the IgG subclasses, IgG3 activates complement more efficiently than does IgG1, while IgG2 is a weak activator and there is no good evidence for complement activation by IgG4 [36]. IgA does probably not activate complement. Despite this, however, IgA deposition on RBCs can lead to fulminant hemolysis [23,37]. A probable explanation is involvement of IgM even in some cases where only IgG or IgA is detected, since IgM will often detach from the RBC before it can be detected by DAT [25]. Upon complement activation in w-AIHA, phagocytosis of C3b-opsonized erythrocytes by reticulo-endothelial cells in the liver is responsible for most of the hemolysis, while full-blown intravascular hemolysis mediated by the terminal complement pathway is usually not prominent [4,7,35]. The explanation is probably the modest activation of the complement pathway, combined with the protective effect of the physiological cell surface complement inhibitors CD55 and CD59 which, unlike in PNH, are intact in AIHA.
Figure 2 summarizes the pathways of erythrocyte destruction in w-AIHA. In conclusion, complement activation does occur to some extent, at least in a proportion of the patients, but is hardly essential for hemolysis in w-AIHA. DAT positivity for C3 fragments is a marker of complement involvement.
Fig. 2.
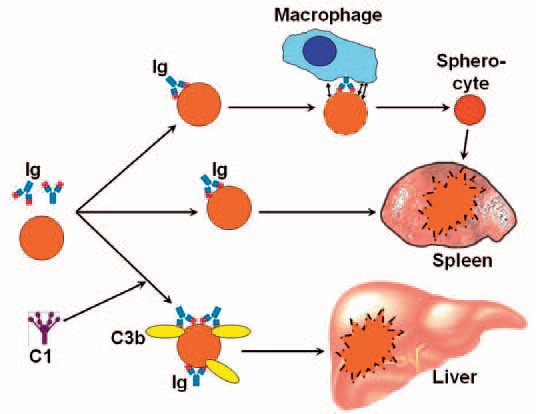
Erythrocyte destruction in warm-antibody mediated autoimmune hemolytic anemia. Explanation: See text. Ig = Immunoglobulin; C = complement. Originally published in BioMed Res Int 2015 [28]. Copyright: S. Berentsen and T. Sundic. Re-used with permission.
Cold Agglutinin Disease
Etiology and Pathogenesis
Primary cold agglutinin disease (CAD) should be distinguished from secondary cold agglutinin syndrome (CAS) [5]. As will be further explained, CAD is a well-defined clinicopathological entity and should, therefore, be called a disease, and not a syndrome [5,38]. Secondary CAS is a syndrome complicating a variety of infectious and neoplastic disorders, and not a well-defined disease. In a Norwegian population-based study, the prevalence of CAD was 16 per million, and the incidence was about 1 per million per year, making CAD account for approximately 15% of AIHA [1,2,39].
Cold agglutinins (CA) are autoantibodies that agglutinate RBCs with a temperature optimum of 3-4 °C but may also act in a warmer environment, depending of the thermal amplitude of the CA [5,40]. If the thermal amplitude exceeds 28-30 °C, the CA will be pathogenic. Low-affinity CA also occur in many healthy individuals; these non-pathogenic CA are polyclonal, have low thermal amplitude and are present in low titers, not higher than 256 and usually lower than 64. More than 90% of pathogenic CA are of the IgM class, and these IgM macromolecules can be pentameric or hexameric [39,41,42].
In general, monoclonal CA are more pathogenic than polyclonal CA, and hexameric IgM is more pathogenic than pentameric IgM [5,42,43]. It has been known for decades that in patients with CAD, IgM antibodies with CA activity are monoclonal and, in more than 90% of the patients, show kappa light chain restriction [44]. Accordingly, CAD patients must have a clonal B-cell lymphoproliferative disorder which has not been fully elucidated until the last years. Two large, retrospective studies of consecutive patients with primary CAD found signs of a bone marrow clonal lymphoproliferation in most patients, but in both series the individual hematological and histological diagnoses showed a striking heterogeneity [39,45]. In one of the series, lymphoplasmacytic lymphoma (LPL) was the most frequent finding, while marginal zone lymphoma (MZL), unclassified clonal lymphoproliferation, and reactive lymphocytosis were also frequently reported [39]. The explanation for this perceived heterogeneity was probably revealed by a recent study in which bone marrow biopsy samples and aspirates from 54 patients with CAD were systematically re-examined by a group of lymphoma pathologists, using a standardized panel of morphological, immunohistochemical, flow-cytometric and molecular methods [38]. The bone marrow findings in these patients were consistent with a surprisingly homogeneous disorder termed ‘primary CA-associated lymphoproliferative disease’ by the authors and distinct from LPL, MZL, and other previously recognized lymphoma entities. The MYD88 L265P somatic mutation, typical for LPL, could not be detected in the samples from patients with CAD [38,46].
Role of Complement in CAD
CA are usually directed against the Ii blood group system, most CA in CAD being specific for the I carbohydrate antigen [47,48,49]. Cooling of blood during passage through acral parts of the circulation allows CA to bind to erythrocytes and cause agglutination (figure 3). Being a strong complement activator, antigen-bound IgM CA on the cell surface binds C1q and thereby initiates the classical complement pathway [8,49,50,51]. C1 esterase activates C4 and C2, generating C3 convertase which results in the cleavage of C3 to C3a and C3b. Upon returning to central parts of the body with a temperature of 37 °C, IgM CA detaches from the cell surface, allowing agglutinated erythrocytes to separate, while C3b remains bound. A proportion of the C3b-coated cells is sequestered by macrophages of the reticulo-endothelial system, mainly in the liver. On the surface of the surviving erythrocytes, C3b is cleaved, leaving high numbers of C3d molecules on the cell surface. These mechanisms explain why the monospecific DAT is strongly positive for C3d in patients with CA-mediated hemolysis and, in the majority, negative for IgM and IgG [39].
Fig. 3.
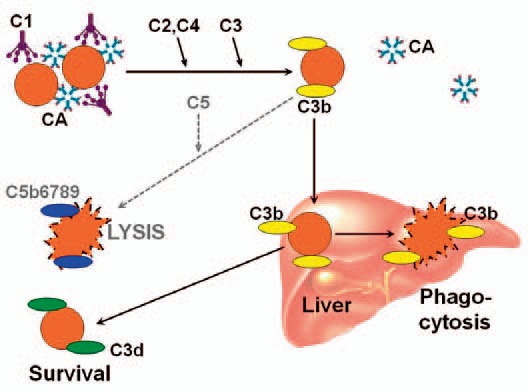
Complement-mediated hemolysis in cold agglutinin disease (CAD) and cold agglutinin syndrome (CAS). Explanation: See text. CA = Cold agglutinin; C = complement. Originally published in BioMed Res Int 2015 [28]. Copyright: S. Berentsen and T. Sundic. Re-used with permission.
Complement activation may proceed beyond the C3b formation step, resulting in C5 activation, formation MAC, and intravascular hemolysis. Due to surface-bound regulatory proteins such as CD55 and CD59, however, the complement activation is usually not sufficient to produce clinically significant activation of the terminal complement pathway. The major mechanism of hemolysis in stable disease, therefore, is the extravascular destruction of C3b-coated erythrocytes [10,35,50]. Obviously, however, C5-mediated intravascular hemolysis does occur in severe acute exacerbations and in some profoundly hemolytic patients, as evidenced by the finding of hemoglobinuria in 15% of the patients and the observation of a beneficial effect of C5 inhibition in at least a couple of patients [39,45,51,52].
Febrile infections, major trauma, or major surgery can result in acute exacerbation of hemolytic anemia in at least two-thirds of patients with CAD [39,51,53]. The explanation for this paradoxical exacerbation is that during steady-state chronic disease, most patients are complement-depleted with low levels of C3 and often undetectable levels of C4. During acute phase reactions, C3 and C4 are repleted and exacerbation of complement-induced hemolysis ensues [48,51].
Secondary Cold Agglutinin Syndrome
Among 295 consecutive individuals with AIHA described retrospectively by Dacie [2] in a single-center series, 7 patients (2.4%) were classified as having CAS secondary to malignant disease. CAS has been described in patients diagnosed with diffuse large B-cell lymphoma, Hodgkin's lymphoma, carcinomas, sarcomas, metastatic melanoma, and chronic myeloproliferative disorders [1]. Some of these associations have been poorly documented [5], and the most convincing association with malignant disease has been described with non-Hodgkin's lymphoma [54,55,56,57]. In CAS complicating aggressive lymphoma, the CA are monoclonal, most often IgM, and have anti-I specificity. The light chain restriction can be lambda as well as kappa [54,57].
Polyclonal anti-I-specific CA of the IgM class are produced as part of the physiological immune response in Mycoplasma pneumoniae pneumonia. They do usually not give rise to significant hemolysis. In a few patients, however, production of high-titer, high-thermal amplitude CA results in hemolytic anemia which is transient but can be severe [5,58,59]. CAS complicating Mycoplasma pneumoniae infection has been reported to account for approximately 8% of AIHA [2]. Still more uncommon but less severe, polyclonal anti-i specific CA of the IgM or IgG class can result in CAS in Epstein-Barr virus infection [5,60]. Transient CAS has also been described following cytomegalovirus infection, varicella, rubella, adenovirus infection, influenza A, Legionella pneumophilica pneumonia, listeriosis and pneumonia caused by Chlamydia species [5]. In CAS secondary to infection or aggressive lymphoma, the erythrocyte breakdown is complement-dependent, mediated by exactly the same mechanisms as in primary CAD (fig. 3) [5,7].
Paroxysmal Cold Hemoglobinuria
In paroxysmal cold hemoglobinuria (PCH), polyclonal cold-reactive IgG antibodies bind to the RBC surface protein antigen termed P but does not agglutinate the erythrocytes. The resulting hemolysis is entirely complement-dependent, and the temperature optimum for complement activation is at 37 °C [61,62]. Such biphasic antibodies are called Donath-Landsteiner hemolysins. In the Donath-Landsteiner's test, one sample of patient blood is incubated at 4 °C and then at 37 °C, while another sample is incubated at 37 °C without having been pre-incubated in the cold [61,62]. If biphasic autoantibodies are present, hemolysis will be observed only in the sample pre-incubated at 4 °C. The sensitivity is limited because the patient blood is often complement-depleted; and in more sensitive modifications of the test, complement is added and/or papain-pretreated RBCs are used [62].
50-100 years ago, PCH was associated with tertiary syphilis, but this form is hardly seen anymore. In the 21th century, PCH occurs almost exclusively in children and accounts for 1-5% of childhood AIHA, making it a rare disease [63]. It appears as an acute, postinfectious complication - in most cases following a virus infection [62]. Single cases have also been reported in Haemophilus influenzae infection and visceral leishmaniasis [63,64].
The P-anti-P complex is a very strong complement activator, resulting in full-blown activation of the classical and terminal pathways (fig. 4). The hemolysis, therefore, is intravascular and massive; the onset is usually sudden, and the clinical features include fever, pallor, jaundice, severe anemia, and macroscopic hemoglobinuria [62,64]. Even though PCH is a transient complication with good prognosis, most patients will need transfusions, which can safely be given provided the same precautions are undertaken as in other cold-antibody AIHA [5].
Fig. 4.
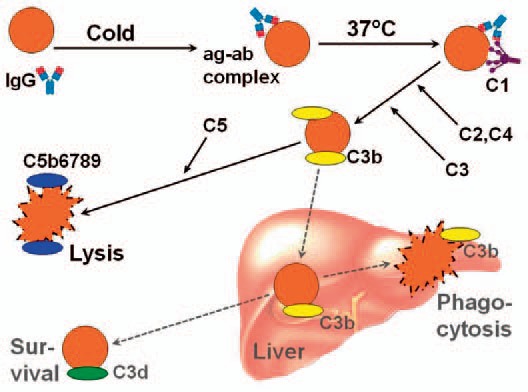
Biphasic, complement-mediated hemolysis in paroxysmal cold hemoglobinuria (PCH). Explanation: See text. Ig = Immunoglobulin; ag = antigen; ab = antibody; C = complement. Originally published in BioMed Res Int 2015 [28]. Copyright: S. Berentsen and T. Sundic. Re-used with permission.
Established Therapies
Established therapies for w-AIHA has been extensively reviewed elsewhere [3,4]. The cornerstone of such therapy is unspecific immunosuppression and/or B-lymphocyte suppression [65] in addition to treatment of any underlying or associated disorder.
In primary CAD, rituximab monotherapy has yielded about 50% response rates and a median 1-year response duration according to two prospective trials [66,67]. Combination therapy for CAD with rituximab and fludarabine in order to target the pathogenic B-cell clone even more efficiently resulted in a 75% response rate, 20% complete responses according to strict criteria and an impressive median response duration of more than 66 months, however with some toxicity [68]. Single case observations with bendamustin- or bortezomib-based therapies as alternative ways of targeting the lymphoproliferative bone marrow disease have reported favorable outcomes [69,70].
For secondary CAS as well as PCH, no documented therapy exists apart from treating the underlying disease when relevant and feasible [5,62].
Therapeutic Complement Modulation
Candidate Substances, Experimental Studies, and Case Observations
The potential of complement modulation for the treatment of AIHA will depend on i) the type of AIHA and extent and level of complement involvement, ii) the availability, safety and efficacy of complement-modulating drugs, and iii) the specific level of complement inhibition by these drugs. The search for targeted, therapeutic inhibitors of the complement cascade has been going on for 30-40 years, with relatively few examples of success so far [9]. Novel in vitro and in vivo models for testing the impact of specific complement inhibition on immune hemolysis are, however, still being developed [10,71].
C1-esterase inhibitor (C1-INH) has been available for decades and is being successfully used for the treatment of hereditary angioedema (HAE) [72]. Although not a complement-mediated disorder, HAE is caused by lack or deficiency of endogenous C1-INH, and replacement therapy has been well studied. In AIHA, on the other hand, endogenous C1-INH production is normal, indicating that physiological concentrations of the inhibitor will not block complement-mediated hemolysis.
Eculizumab, a humanized monoclonal C5 antibody, blocks the terminal pathway and, thereby, prevents intravascular hemolysis by MAC. Therapy with eculizumab has been a great success in PNH, although complement-mediated hemolysis is not completely prevented [73]. The explanation for this is probably that patients with PNH lack physiological inhibitors both at a downstream level in the terminal pathway (CD59) and at an upstream level in the classical pathway (CD55). In consequence, a slight to moderate hemolysis mediated by phagocytosis of C3b-opsonized erythrocytes will still occur along the same pathway as described in CAD, independent of C5 activation or inhibition [12].
Some newer complement-modulating drugs have been studied with promising results in preclinical experiments but not yet in the in vivo setting. Compstatin Cp40 is a low-molecular-weight peptide complement inhibitor that blocks cleavage of C3 and has been found to efficiently prevent lysis of erythrocytes from PNH patients in vitro [74]. Peptide inhibitor of C1 (PIC1) is a recently described class of small molecules that targets C1q, blocking the activation of associated serine proteases (C1s-C1r-C1r-C1s) and subsequent classical pathway activation [75]. PIC1 has been studied in acute hemolytic transfusion reactions in animals but, so far, not in AIHA-related experiments.
TNT003, a mouse monoclonal anti-C1s antibody, has recently been shown to completely inhibit in vitro hemolysis induced by CA [10]. This antibody targets C1s serine protease activity. Using CA samples from 40 patients with CAD, the authors found that TNT003 prevented CA-induced deposition of C3 fragments on the RBC at the same concentration of antibody that stopped hemolysis. Furthermore, C1s inhibition by TNT003 resulted in prevention of in vitro erythrophagocytosis by a phagocytic cell line. The classical-pathway-driven production of the anaphylotoxins C4a, C3a and C5a was also inhibited [10]. Favorable in vitro results have also been described with TNT003's humanized counterpart: TNT009 [76].
Future Perspective
As shown above, complement activation plays a role in w-AIHA but is not essential for pathogenesis in most patients. Complement modulation may be expected, therefore, to be of limited therapeutic value in w-AIHA in general and of no value if DAT is negative for C3 fragments. Wouters and colleagues [77] described, however, a favorable effect of plasma-derived C1-INH in a patient with a C3d-positive, therapy-resistant severe w-AIHA secondary to an aggressive non-Hodgkin's lymphoma. Although very high doses of C1-INH were required, hemolysis was efficiently controlled, and the efficacy of erythrocyte transfusion dramatically improved following treatment. No other clinical observations on the results of complement inhibition have been published in w-AIHA. In patients with a positive DAT for C3d and very severe hemolysis, further studies of complement inhibition even at a more downstream level would be of interest, mainly as an attempt to temporarily control hemolysis.
Given that hemolysis in CAD is entirely complement-dependent, studies of complement inhibition would be relevant in CAD. A case report by Röth and colleagus [52] described a favorable effect of therapy with eculizumab. This observation may seem somewhat surprising, since the predominant hemolytic pathway in CAD is not C5/MAC-mediated. A probable explanation is that activation of the terminal complement pathway does occur, after all, in acute exacerbations, in the chronic state of some severely affected patients, and, possibly, as a minor pathway even in less severely affected patients. Further studies will be of interest.
In theory, complement inhibition at the C1 level should be very promising in CAD because this will block the classical-pathway-dependent, C3b-mediated extravascular hemolysis without compromising the alternative and lectin complement pathways. The published in vitro studies of TNT003 and TNT009 are highly interesting therefore, and it is to be hoped that such a monoclonal antibody can be developed and further tested in the preclinical and clinical setting [10,11,75].
Given that immunochemotherapy directed at the pathogenic B-cell clone is efficient and requires administration only for a limited period of time, do we actually need complement-modulating therapies for CAD? First, in at least 25% of the patients, immunochemotherapy is unsuccessful because of treatment failure or toxicity [68]. Second, rapidly acting therapies should be developed for some specific clinical situations, for example acute severe exacerbations induced by infections, trauma or major surgery, and, possibly, before cardiac surgery in selected patients.
In the uncommon cases of CAS secondary to specific infection, there is often no need for therapy for the CAS per se. However, this is not always the case. Particularly in CAS following Mycoplasma pneumoniae pneumonia, the patients can be profoundly anemic and transfusion-dependent for weeks until spontaneous resolution occurs [5]. Clinicians and patients would welcome a possibility for temporary control of this situation by complement inhibition along the same lines that may be developed in primary CAD. Systematic studies would be interesting but probably difficult to perform because of the rarity of the disorder.
In the rare cases of post-infectious PCH in children, measures for temporary control of the hemolysis will be valuable if such therapies can be developed. Since the terminal complement pathway is heavily involved, we do not necessarily need new substances; exploring the efficacy of eculizumab would be of great interest. Probably, however, prospective trials will never be performed because there are too few patients for such studies.
Present and future possibilities for therapeutic complement inhibition in AIHA are summarized in figure 5. It is important to ask whether such therapy will be dangerous. The complement system is, after all, an essential part of the innate immune system. Based on studies of eculizumab in PNH, we already have extensive information on the risk of severe infection following C5 inhibition. Provided the patients can be efficiently protected against meningococci, studies and clinical experience have shown that the risk of infection is negligible [73]. Complement inhibition at the C3 level may carry a much higher risk because efficient inhibition of C3 will completely block complement activation beyond this level, whether initiated by the classical, alternative or lectin pathway [11,14,74]. Interestingly, however, the still more proximal blockade at the C1 level achieved by TNT003 will selectively affect the classical pathway as required for control of hemolysis in CAD, while the lectin and alternative pathways will remain intact. Probably therefore, these pathways probably will still enable the system to generate the anaphylotoxins C3a and C5a in response to microbial stimuli, even though the production of these anaphylotoxins induced by the classical pathway will be blocked [10,11,14]. Although this selectivity may, theoretically, reduce the risk of infection, careful studies will be required to address this issue.
Fig. 5.
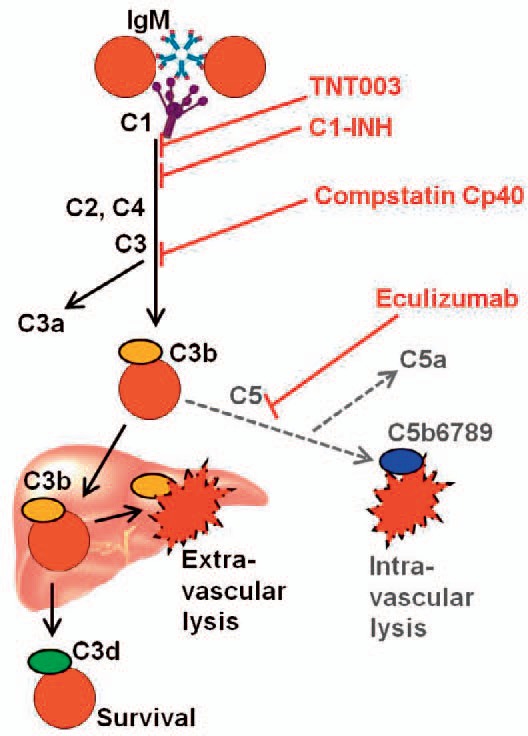
Candidate complement modulators and possible levels of therapeutic complement inhibition in autoimmune hemolytic anemias. Explanation: See text. Ig = Immunoglobulin; C = complement, C1-INH = C1 inhibitor. First published in Blood 2014 [11]. Copyright: Blood, the Journal of the American Society of Hematology. Re-used with permission.
Disclosure Statement
S. Berentsen has received research support from Mundipharma and lecture honoraria and travel grants from Alexion.
References
- 1.Sokol RJ, Hewitt S, Stamps BK. Autoimmune haemolysis: an 18-year study of 865 cases referred to a regional transfusion centre. Br Med J (Clin Res Ed) 1981;282:2023–2027. doi: 10.1136/bmj.282.6281.2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dacie J. The auto-immune haemolytic anaemias: introduction; in Dacie J (ed): The Haemolytic Anaemias, Vol. 3. London, Churchill Livingstone, 1992, pp 1-5.
- 3.Michel M. Classification and therapeutic approaches in autoimmune hemolytic anemia: an update. Expert Rev Hematol. 2011;4:607–618. doi: 10.1586/ehm.11.60. [DOI] [PubMed] [Google Scholar]
- 4.Packman CH. Hemolytic anemia due to warm autoantibodies. Blood Rev. 2008;22:17–31. doi: 10.1016/j.blre.2007.08.001. [DOI] [PubMed] [Google Scholar]
- 5.Berentsen S, Tjonnfjord GE. Diagnosis and treatment of cold agglutinin mediated autoimmune hemolytic anemia. Blood Rev. 2012;26:107–115. doi: 10.1016/j.blre.2012.01.002. [DOI] [PubMed] [Google Scholar]
- 6.Garratty G. The James Blundell Award Lecture 2007: do we really understand immune red cell destruction? Transfus Med. 2008;18:321–334. doi: 10.1111/j.1365-3148.2008.00891.x. [DOI] [PubMed] [Google Scholar]
- 7.Dacie J. Auto-immune haemolytic anaemia (AIHA): pathogenesis; in Dacie J (ed): The Haemolytic Anaemias, Vol. 3. London. Churchill Livingstone, 1992, pp 392-451.
- 8.Berentsen S, Beiske K, Tjonnfjord GE. Primary chronic cold agglutinin disease: an update on pathogenesis, clinical features and therapy. Hematology. 2007;12:361–370. doi: 10.1080/10245330701445392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Larghi EL, Kaufman TS. Modulators of complement activation a patent review (2008-2013) Expert Opin Ther Pat. 2014;24:665–686. doi: 10.1517/13543776.2014.898063. [DOI] [PubMed] [Google Scholar]
- 10.Shi J, Rose EL, Singh A, Hussain S, Stagliano NE, Parry GC, et al. TNT003, an inhibitor of the serine protease C1s, prevents complement activation induced by cold agglutinin disease patient autoantibodies. Blood. 2014;123:4015–4022. doi: 10.1182/blood-2014-02-556027. [DOI] [PubMed] [Google Scholar]
- 11.Berentsen S. Complement, cold agglutinins, and therapy. Blood. 2014;123:4010–4012. doi: 10.1182/blood-2014-04-568733. [DOI] [PubMed] [Google Scholar]
- 12.Hill A, Rother RP, Arnold L, Kelly R, Cullen MJ, Richards SJ, et al. Eculizumab prevents intravascular hemolysis in patients with paroxysmal nocturnal hemoglobinuria and unmasks low-level extravascular hemolysis occurring through C3 opsonization. Haematologica. 2010;95:567–573. doi: 10.3324/haematol.2009.007229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brodsky RA. Paroxysmal nocturnal hemoglobinuria. Blood. 2014;124:2804–2811. doi: 10.1182/blood-2014-02-522128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Murphy K. Janeway's Immunobiology, 8th ed. New York, Garland, 2011.
- 15.Schreiber AD, Frank MM. Role of antibody and complement in the immune clearance and destruction of erythrocytes. I. In vivo effects of IgG and IgM complement-fixing sites. J Clin Invest. 1972;51:575–582. doi: 10.1172/JCI106846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Berentsen S, Randen U, Tjonnfjord GE. Cold agglutinin-mediated autoimmune hemolytic anemia. Hematol Oncol Clin North Am. 2015;29:455–471. doi: 10.1016/j.hoc.2015.01.002. [DOI] [PubMed] [Google Scholar]
- 17.Chen M, Daha MR, Kallenberg CG. The complement system in systemic autoimmune disease. J Autoimmun. 2010;34:J276–286. doi: 10.1016/j.jaut.2009.11.014. [DOI] [PubMed] [Google Scholar]
- 18.Gehrs BC, Friedberg RC. Autoimmune hemolytic anemia. Am J Hematol. 2002;69:258–271. doi: 10.1002/ajh.10062. [DOI] [PubMed] [Google Scholar]
- 19.Diehl LF, Ketchum LH. Autoimmune disease and chronic lymphocytic leukemia: autoimmune hemolytic anemia, pure red cell aplasia, and autoimmune thrombocytopenia. Semin Oncol. 1998;25:80–97. [PubMed] [Google Scholar]
- 20.Fagiolo E. Immunological tolerance loss vs. erythrocyte self antigens and cytokine network disregulation in autoimmune hemolytic anaemia. Autoimmun Rev. 2004;3:53–59. doi: 10.1016/S1568-9972(03)00085-5. [DOI] [PubMed] [Google Scholar]
- 21.Ward FJ, Hall AM, Cairns LS, Leggat AS, Urbaniak SJ, Vickers MA, et al. Clonal regulatory T cells specific for a red blood cell autoantigen in human autoimmune hemolytic anemia. Blood. 2008;111:680–687. doi: 10.1182/blood-2007-07-101345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hoffman PC. Immune hemolytic anemia - selected topics. Hematology Am Soc Hematol Educ Program 2006;13-18. [DOI] [PubMed]
- 23.Janvier D, Sellami F, Missud F, Fenneteau O, Vilmer E, Cartron J, et al. Severe autoimmune hemolytic anemia caused by a warm IgA autoantibody directed against the third loop of band 3 (RBC anion-exchange protein 1) Transfusion. 2002;42:1547–1552. doi: 10.1046/j.1537-2995.2002.00235.x. [DOI] [PubMed] [Google Scholar]
- 24.Arndt PA, Leger RM, Garratty G. Serologic findings in autoimmune hemolytic anemia associated with immunoglobulin M warm autoantibodies. Transfusion. 2009;49:235–242. doi: 10.1111/j.1537-2995.2008.01957.x. [DOI] [PubMed] [Google Scholar]
- 25.Zeerleder S. Autoimmune haemolytic anaemia - a practical guide to cope with a diagnostic and therapeutic challenge. Neth J Med. 2011;69:177–184. [PubMed] [Google Scholar]
- 26.Sachs UJ, Roder L, Santoso S, Bein G. Does a negative direct antiglobulin test exclude warm autoimmune haemolytic anaemia? A prospective study of 504 cases. Br J Haematol. 2006;132:655–656. doi: 10.1111/j.1365-2141.2005.05955.x. [DOI] [PubMed] [Google Scholar]
- 27.Gilsanz F, De La Serna J, Moltó L, Alvarez-Mon M. Hemolytic anemia in chronic large granular lymphocytic leukemia of natural killer cells: cytotoxicity of natural killer cells against autologous red cells is associated with hemolysis. Transfusion. 1996;36:463–466. doi: 10.1046/j.1537-2995.1996.36596338025.x. [DOI] [PubMed] [Google Scholar]
- 28.Berentsen S, Sundic T. Red blood cell destruction in autoimmune hemolytic anemia: role of complement and potential new targets for therapy. Biomed Res Int. 2015;2015:363278. doi: 10.1155/2015/363278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jandl JH, Jones AR, Castle WB. The destruction of red cells by antibodies in man. I. Observations of the sequestration and lysis of red cells altered by immune mechanisms. J Clin Invest. 1957;36:1428–1459. doi: 10.1172/JCI103542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jandl JH, Kaplan ME. The destruction of red cells by antibodies in man. III. Quantitative factors influencing the patterns of hemolysis in vivo. J Clin Invest. 1960;39:1145–1156. doi: 10.1172/JCI104129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kurlander RJ, Rosse WF. Monocyte-mediated destruction in the presence of serum of red cells coated with antibody. Blood. 1979;54:1131–1139. [PubMed] [Google Scholar]
- 32.LoBuglio AF, Cotran RS, Jandl JH. Red cells coated with immunoglobulin G: binding and sphering by mononuclear cells in man. Science. 1967;158:1582–1585. doi: 10.1126/science.158.3808.1582. [DOI] [PubMed] [Google Scholar]
- 33.Abramson N, Lo Buglio AF, Jandl JH, Cotran RS. The interaction between human monocytes and red cells. Binding characteristics. J Exp Med. 1970;132:1191–1206. doi: 10.1084/jem.132.6.1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Meulenbroek EM, Wouters D, Zeerleder S. Methods for quantitative detection of antibody-induced complement activation on red blood cells. J Vis Exp. 2014;(83):e51161. doi: 10.3791/51161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kurlander RJ, Rosse WF, Logue GL. Quantitative influence of antibody and complement coating of red cells on monocyte-mediated cell lysis. J Clin Invest. 1978;61:1309–1319. doi: 10.1172/JCI109048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Abramson N, Gelfand EW, Jandl JH, Rosen FS. The interaction between human monocytes and red cells. Specificity for IgG subclasses and IgG fragments. J Exp Med. 1970;132:1207–1215. doi: 10.1084/jem.132.6.1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bardill B, Mengis C, Tschopp M, Wuillemin WA. Severe IgA-mediated auto-immune haemolytic anaemia in a 48-yr-old woman. Eur J Haematol. 2003;70:60–63. doi: 10.1034/j.1600-0609.2003.02846.x. [DOI] [PubMed] [Google Scholar]
- 38.Randen U, Troen G, Tierens A, Steen C, Warsame A, Beiske K, et al. Primary cold agglutinin-associated lymphoproliferative disease: a B-cell lymphoma of the bone marrow distinct from lymphoplasmacytic lymphoma. Haematologica. 2014;99:497–504. doi: 10.3324/haematol.2013.091702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Berentsen S, Ulvestad E, Langholm R, Beiske K, Hjorth-Hansen H, Ghanima W, et al. Primary chronic cold agglutinin disease: a population based clinical study of 86 patients. Haematologica. 2006;91:460–466. [PubMed] [Google Scholar]
- 40.Rosse WF, Adams JP. The variability of hemolysis in the cold agglutinin syndrome. Blood. 1980;56:409–416. [PubMed] [Google Scholar]
- 41.Harboe M, Deverill J. Immunochemical properties of cold haemagglutinins. Scand J Haematol. 1964;61:223–237. doi: 10.1111/j.1600-0609.1964.tb00019.x. [DOI] [PubMed] [Google Scholar]
- 42.Hughey CT, Brewer JW, Colosia AD, Rosse WF, Corley RB. Production of IgM hexamers by normal and autoimmune B cells: implications for the physiologic role of hexameric IgM. J Immunol. 1998;161:4091–4097. [PubMed] [Google Scholar]
- 43.Stone MJ, McElroy YG, Pestronk A, Reynolds JL, Newman JT, Tong AW. Human monoclonal macroglobulins with antibody activity. Semin Oncol. 2003;30:318–324. doi: 10.1053/sonc.2003.50077. [DOI] [PubMed] [Google Scholar]
- 44.Harboe M, van Furth R, Schubothe H, Lind K, Evans RS. Exclusive occurrence of K chains in isolated cold haemagglutinins. Scand J Haematol. 1965;2:259–266. doi: 10.1111/j.1600-0609.1965.tb01303.x. [DOI] [PubMed] [Google Scholar]
- 45.Swiecicki PL, Hegerova LT, Gertz MA. Cold agglutinin disease. Blood. 2013;122:1114–1121. doi: 10.1182/blood-2013-02-474437. [DOI] [PubMed] [Google Scholar]
- 46.Treon SP, Xu L, Yang G, Zhou Y, Liu X, Cao Y, et al. MYD88 L265P somatic mutation in Waldenström's macroglobulinemia. N Engl J Med. 2012;367:826–833. doi: 10.1056/NEJMoa1200710. [DOI] [PubMed] [Google Scholar]
- 47.Issitt PD. I blood group system and its relationship to disease. J Med Lab Tech. 1968;25:1–6. [PubMed] [Google Scholar]
- 48.Ulvestad E, Berentsen S, Bo K, Shammas FV. Clinical immunology of chronic cold agglutinin disease. Eur J Haematol. 1999;63:259–266. doi: 10.1111/j.1600-0609.1999.tb01887.x. [DOI] [PubMed] [Google Scholar]
- 49.Jonsen J, Kass E, Harboe M. Complement and complement components in acquired hemolytic anemia with high titer cold antibodies. Acta Med Scand. 1961;170:725–729. doi: 10.1111/j.0954-6820.1961.tb00291.x. [DOI] [PubMed] [Google Scholar]
- 50.Jaffe CJ, Atkinson JP, Frank MM. The role of complement in the clearance of cold agglutinin-sensitized erythrocytes in man. J Clin Invest. 1976;58:942–949. doi: 10.1172/JCI108547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ulvestad E, Berentsen S, Mollnes TE. Acute phase haemolysis in chronic cold agglutinin disease. Scand J Immunol. 2001;54:239–242. doi: 10.1046/j.1365-3083.2001.00960.x. [DOI] [PubMed] [Google Scholar]
- 52.Roth A, Huttmann A, Rother RP, Duhrsen U, Philipp T. Long-term efficacy of the complement inhibitor eculizumab in cold agglutinin disease. Blood. 2009;113:3885–3886. doi: 10.1182/blood-2009-01-196329. [DOI] [PubMed] [Google Scholar]
- 53.Ulvestad E. Paradoxical haemolysis in a patient with cold agglutinin disease. Eur J Haematol. 1998;60:93–100. doi: 10.1111/j.1600-0609.1998.tb01004.x. [DOI] [PubMed] [Google Scholar]
- 54.Crisp D, Pruzanski W. B-cell neoplasms with homogeneous cold-reacting antibodies (cold agglutinins) Am J Med. 1982;72:915–922. doi: 10.1016/0002-9343(82)90852-x. [DOI] [PubMed] [Google Scholar]
- 55.Niainle F, Hamnvik OP, Gulmann C, Bermingham C, Kelly J, Mc EP, et al. Diffuse large B-cell lymphoma with isolated bone marrow involvement presenting with secondary cold agglutinin disease. Int J Lab Hematol. 2008;30:444–445. doi: 10.1111/j.1751-553X.2007.00977.x. [DOI] [PubMed] [Google Scholar]
- 56.Eskazan AE, Akmurad H, Ongoren S, Ozer O, Ferhanoglu B. Primary gastrointestinal diffuse large B cell lymphoma presenting with cold agglutinin disease. Case Rep Gastroenterol. 2011;5:262–266. doi: 10.1159/000328445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Dacie J. Haemolytic anaemias associated with malignant lymphomas other than Hodgkin's disease and chronic lymphocytic leukaemia (CLL); in Dacie J (ed): The Haemolytic Anaemias, Vol. 4. London, Churchill Livingstone, 1995, pp 27-40.
- 58.Linz DH, Tolle SW, Elliot DL. Mycoplasma pneumoniae pneumonia. Experience at a referral center. West J Med. 1984;140:895–900. [PMC free article] [PubMed] [Google Scholar]
- 59.Ginsberg HS. Acute hemolytic anemia in primary atypical pneumonia associated with high titer of cold agglutinins; report of a case. N Engl J Med. 1946;234:826–829. doi: 10.1056/nejm194606202342503. [DOI] [PubMed] [Google Scholar]
- 60.Wentworth P, Bate LR. Acute hemolytic anemia secondary to infectious mononucleosis. Can Med Assoc J. 1980;123:482–486. [PMC free article] [PubMed] [Google Scholar]
- 61.Donath J, Landsteiner K. Über Paroxysmale Hämoglobinurie. Münch M Wochenschr. 1904;51:1590–1593. [Google Scholar]
- 62.Gertz MA. Cold hemolytic syndrome. Hematology Am Soc Hematol Educ Program 2006;19-23. [DOI] [PubMed]
- 63.Ziman A, Hsi R, Goldfinger D. Transfusion medicine illustrated: Donath-Landsteiner antibody-associated hemolytic anemia after Haemophilus influenzae infection in a child. Transfusion. 2004;44:1127–1128. doi: 10.1111/j.1537-2995.2004.03403.x. [DOI] [PubMed] [Google Scholar]
- 64.D'Angiò M, Ceglie T, Giovanetti G, Neri A, Santilio I, Nunes V, et al. Visceral leishmaniasis presenting with paroxysmal cold haemoglobinuria. Blood Transfus. 2014;12(suppl 1):s141–s143. doi: 10.2450/2013.0034-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Birgens H, Frederiksen H, Hasselbalch HC, Rasmussen IH, Nielsen OJ, Kjeldsen L, et al. A phase III randomized trial comparing glucocorticoid monotherapy versus glucocorticoid and rituximab in patients with autoimmune haemolytic anaemia. Br J Haematol. 2013;163:393–399. doi: 10.1111/bjh.12541. [DOI] [PubMed] [Google Scholar]
- 66.Berentsen S, Ulvestad E, Gjertsen BT, Hjorth-Hansen H, Langholm R, Knutsen H, et al. Rituximab for primary chronic cold agglutinin disease: a prospective study of 37 courses of therapy in 27 patients. Blood. 2004;103:2925–2928. doi: 10.1182/blood-2003-10-3597. [DOI] [PubMed] [Google Scholar]
- 67.Schollkopf C, Kjeldsen L, Bjerrum OW, Mourits-Andersen HT, Nielsen JL, Christensen BE, et al. Rituximab in chronic cold agglutinin disease: a prospective study of 20 patients. Leuk Lymphoma. 2006;47:253–260. doi: 10.1080/10428190500286481. [DOI] [PubMed] [Google Scholar]
- 68.Berentsen S, Randen U, Vagan AM, Hjorth-Hansen H, Vik A, Dalgaard J, et al. High response rate and durable remissions following fludarabine and rituximab combination therapy for chronic cold agglutinin disease. Blood. 2010;116:3180–3184. doi: 10.1182/blood-2010-06-288647. [DOI] [PubMed] [Google Scholar]
- 69.Gueli A, Gottardi D, Hu H, Ricca I, De Crescenzo A, Tarella C. Efficacy of rituximab-bendamustine in cold agglutinin haemolytic anaemia refractory to previous chemo-immunotherapy: a case report. Blood Transfus. 2013;11:311–314. doi: 10.2450/2012.0166-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Carson KR, Beckwith LG, Mehta J. Successful treatment of IgM-mediated autoimmune hemolytic anemia with bortezomib. Blood. 2010;115:915. doi: 10.1182/blood-2009-09-242917. [DOI] [PubMed] [Google Scholar]
- 71.Shah TA, Mauriello CT, Hair PS, Sharp JA, Kumar PS, Lattanzio FA, et al. Complement inhibition significantly decreases red blood cell lysis in a rat model of acute intravascular hemolysis. Transfusion. 2014;54:2892–2900. doi: 10.1111/trf.12695. [DOI] [PubMed] [Google Scholar]
- 72.Bork K, Steffensen I, Machnig T. Treatment with C1-esterase inhibitor concentrate in type I or II hereditary angioedema: a systematic literature review. Allergy Asthma Proc. 2013;34:312–327. doi: 10.2500/aap.2013.34.3677. [DOI] [PubMed] [Google Scholar]
- 73.Hillmen P, Young NS, Schubert J, Brodsky RA, Socie G, Muus P, et al. The complement inhibitor eculizumab in paroxysmal nocturnal hemoglobinuria. N Engl J Med. 2006;355:1233–1243. doi: 10.1056/NEJMoa061648. [DOI] [PubMed] [Google Scholar]
- 74.Risitano AM, Ricklin D, Huang Y, Reis ES, Chen H, Ricci P, et al. Peptide inhibitors of C3 activation as a novel strategy of complement inhibition for the treatment of paroxysmal nocturnal hemoglobinuria. Blood. 2014;123:2094–2101. doi: 10.1182/blood-2013-11-536573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sharp JA, Whitley PH, Cunnion KM, Krishna NK. Peptide inhibitor of complement C1, a novel suppressor of classical pathway activation: mechanistic studies and clinical potential. Front Immunol. 2014;5:406. doi: 10.3389/fimmu.2014.00406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Panicker S, Shi J, Rose E, Hussain S, Tom S, Strober W. TNT009, a classical complement pathway specific inhibitor, prevents complement dependent hemolysis induced by cold agglutinin disease patient autoantibodies. Presentation at the 55th Meeting of the American Society of Hematology; New Orleans, LA, USA. December 8, 2013: (Paper 64043). https://ash.confex.com/ ash/2013/webprogram/Paper64043.html (last accessed July 28, 2015).
- 77.Wouters D, Stephan F, Strengers P, de Haas M, Brouwer C, Hagenbeek A, et al. C1-esterase inhibitor concentrate rescues erythrocytes from complement-mediated destruction in autoimmune hemolytic anemia. Blood. 2013;121:1242–1244. doi: 10.1182/blood-2012-11-467209. [DOI] [PubMed] [Google Scholar]


