Abstract
AIM: To compare outcomes for patients presenting with stage IV colorectal cancer and an asymptomatic primary tumour, undergoing primary tumour resection (PTR) plus palliative chemotherapy vs primary chemotherapy up-front.
METHODS: A literature search was conducted using MEDLINE and EMBASE. The primary outcome was overall survival. Secondary outcomes included perioperative mortality, morbidity and delayed surgical intervention rates in patients undergoing PTR and subsequent complication rates in patients with an un-resected primary tumour. Tertiary outcomes included impact on systemic treatment and identification of prognostic factors relevant for survival in this cohort.
RESULTS: Twenty non-randomised studies met the inclusion criteria. Eleven studies included comparative overall survival data. Three studies showed an overall survival advantage for PTR, 7 studies showed no statistically significant advantage, and 1 study showed a significant worsening in survival in the surgical group. The perioperative mortality rate ranged from 0% to 8.5%, and post-operative morbidity rate from 10% to 35%, mainly minor complications that did not preclude subsequent chemotherapy. The rate of delayed primary-tumour related symptoms, most commonly obstruction, in patients with an un-resected primary tumour ranged from 3% to 46%. The strongest independent poor prognostic factor was extensive hepatic metastases, in addition to poor performance status, M1b stage and non-use of modern chemotherapy agents.
CONCLUSION: Based on the current literature, both PTR and up front chemotherapy appear appropriate initial management strategies, with a trend towards an overall survival advantage with PTR. The procedure has a low post-operative mortality, and most complications are transient and minor. The results of recruiting randomised trials are eagerly anticipated.
Keywords: Colorectal cancer, Resection, Primary tumour, Asymptomatic, Unresectable metastases, Chemotherapy, Complications
Core tip: The management of asymptomatic primary tumours in stage IV colorectal cancer is under debate. A literature review was performed focusing on this cohort, with patients undergoing primary tumour resection (PTR) vs up front chemotherapy. Survival appears equivalent with both management strategies, with a trend to an advantage in PTR. Surgical mortality is low and most morbidity transient. Most studies are retrospective, small and non-randomised. Larger randomised controlled trials are awaited.
INTRODUCTION
Colorectal cancer is the third most common cancer in men and the second in women worldwide[1]. Approximately 20% of patients present with stage IV disease, and the vast majority (70%-80%) of these patients are incurable. There is no consensus regarding the appropriate management of an asymptomatic or minimally symptomatic primary lesion in these patients. While patients presenting with symptoms suggestive of obstruction, bleeding or perforation are often surgically managed to palliate these acute symptoms, the majority of patients present with systemic symptoms (e.g., weight loss, fatigue, anorexia) and an asymptomatic primary lesion. There are no published randomised controlled trials addressing this clinical question. The CAIRO4[2] and SYNCHRONOUS[3] trials (colon cancer) and GRECCAR-8 trial[4] (rectal cancer) are currently recruiting with results not expected to be available for a number of years.
There is an increasing body of evidence suggesting a survival advantage in patients undergoing primary tumour resection (PTR). This includes post-hoc analyses of randomised trial data[5,6], meta-analyses[7] and population-wide registry data[8]. Improved survival outcomes in advanced disease associated with surgical debulking have a well-established evidence base in epithelial ovarian[9] and renal[10] malignancies. However, most of the currently published evidence relating to colorectal cancer encompasses patients with both symptomatic and asymptomatic primary tumours. More pertinently, most studies include a heterogeneous population, including a significant proportion of patients with poor performance status at diagnosis, who are unfit for PTR. Selection bias may thus skew survival outcomes in favour of the PTR cohort who are likely to be of superior performance status, have fewer co-morbidities, and possibly less burden of disease at diagnosis. Many of the current reviews use data collected in the era prior to routine use of modern chemotherapy regimes and biological agents, including the vascular endothelial growth factor-A monoclonal antibody bevacizumab, and the epidermal growth factor receptor (EGFR) inhibitors cetuximab and panitumumab. These have all had a major impact on survival and therefore it is essential to review patients in this current clinical context.
PTR reduces the risk of subsequent local tumour related complications, primarily obstruction, but also perforation, bleeding and fistulae formation. These complications often warrant emergency surgery, which has a higher rate of peri-operative mortality and morbidity than elective surgery. This may be more problematic when the patient has myelosuppression due to systemic chemotherapy. Any subsequent emergency surgery may also interrupt the use of systemic chemotherapy. This may be a more critical delay later in the course of the patient’s illness as their burden of disease increases. Intact primary tumours may cause systemic complications including weight loss, anorexia, nutritional depletion and pain. They can also cause local complications (diarrhoea, faecal incontinence, etc.) that can impact significantly on quality of life.
Arguments supportive of non-resection strategies up front [primary chemotherapy (PC)] include the risks of post-operative morbidity and mortality. Surgery can delay the use of systemic chemotherapy. Furthermore, the risks of complications from an un-resected primary lesion have been quoted by some to be relatively low[11]. Modern chemotherapy regimes are associated with high response rates, suggesting that chemotherapy may be sufficient to control the primary[12]. A recent Cochrane Collaboration Systematic Review[13] did not find consistently improved outcomes after PTR (although it identified a paucity of sound clinical trials), and current NCCN guidelines support primary resection only in the setting of symptomatic disease[14].
This review was designed to summarise the current literature available, focusing primarily on the outcomes of overall survival, and additional outcomes of perioperative morbidity and mortality, delayed complication rates in both groups, and impact on subsequent chemotherapy. Identification of prognostic markers was also reviewed.
MATERIALS AND METHODS
An extensive literature search was conducted using MEDLINE and EMBASE. Results were limited to 1980-2015 and restricted to English language articles. Search subject headings and MeSH terms included Colorectal Neoplasms, Colon Neoplasms and Rectal Neoplasms, Stage IV, General Surgery, Drug Therapy and the keywords asymp* and symp*. The search strategy was designed to be broad and relevant articles were manually searched to include articles with relevant asymptomatic groups or subgroups. The citations of relevant studies were examined to identify additional articles (Figure 1).
Figure 1.
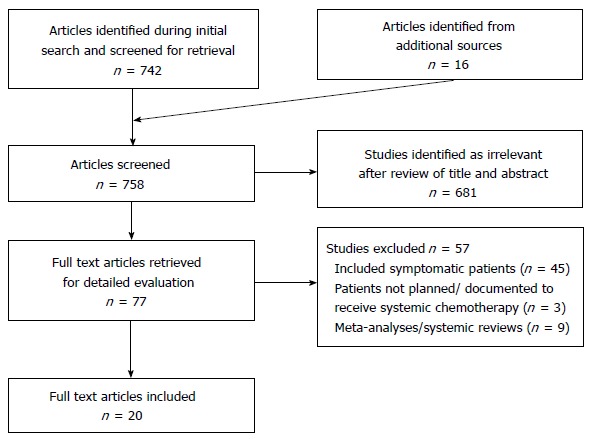
Flow diagram of literature search.
Only studies in which the patients were planned for systemic chemotherapy (after PTR or upfront) were included. Inclusion criteria specified patients with confirmed adenocarcinoma of the colon or rectum, excluding other histological diagnoses. Exclusion criteria included patients undergoing upfront “curative” resection of the primary tumour with staged/simultaneous resection of metastases. Patients undergoing non-resection surgery upfront (including diverting stoma, internal bypass, etc.) were also excluded. For the primary outcome overall survival, all articles were two arm studies in which PTR and PC were compared. For the remaining outcomes, single arm studies involving patients undergoing either PTR or PC were also included.
The primary outcome of interest was overall survival (defined as date of diagnosis to date of death). Survival was determined by the Kaplan-Meier method and distributions compared by the log rank test in all cited articles. The overall significance level was set at 0.05. Secondary outcomes were peri-operative (30 d) mortality, post-operative morbidity (any recorded complication), and delayed surgical intervention for complications in patients undergoing PTR. Other secondary outcomes included the development of delayed primary tumour related symptoms warranting intervention in patients undergoing PC. Tertiary outcomes included the impact of treatment choice on subsequent systemic chemotherapy (timing from diagnosis to chemotherapy, and development of grade 3 or 4 chemotherapy-related toxicities), and prognostic variables influencing overall survival in the asymptomatic cohort, which was determined by multivariate analysis, using the Cox proportional hazards model.
RESULTS
Study characteristics
Twenty studies met the inclusion and exclusion criteria (Table 1). Eleven studies, all retrospective in nature, compared the outcomes of patients undergoing PTR followed by systemic chemotherapy, vs PC upfront. Of these, 1 study included an asymptomatic subgroup within a larger cohort. All of these studies provided overall survival data. A further 7 studies were single-arm studies looking at patients undergoing PC, 4 retrospective and 3 prospective. An additional 2 studies, both retrospective, were single-arm studies following the outcomes of patients undergoing PTR.
Table 1.
Study characteristics
| Ref. | Years data collected | Country | Type of study | Total n | % of group receiving chemo | Predominant chemotherapy regime | Targeted agent use |
| 2 arms: PTR vs PC | (PTR n/PC n) | (PTR/PC) | |||||
| Yun et al[18] (2014) | 2000-2008 | South Korea | Retrospective, propensity-score matched cohort, single centre | 416 (218/198) | 66/100 | Doublet | ND |
| Matsumoto et al[19] (2014) | 2005-2011 | Japan | Retrospective, single centre | 88 (41/47) | 85/100 | Doublet | Approx 50% received targeted agent |
| Ahmed et al[42] (2014) Subgroup | 1992-2005 | Canada | Retrospective, multicentre | 834 | 100/100 | ND | < 2% |
| Cetin et al[22] (2013) | 2006-2010 | Turkey | Retrospective, multi centre | 99 (53/46) | 100/100 | Doublet | 100% received bevacizumab |
| Boselli et al[15] (2013) | 2010-2011 | Italy | Retrospective, single centre | 48 (17/31) | 65/100 | Doublet | > 50% received bevacizumab 1st line |
| Seo et al[20] (2010) | 2001-2008 | South Korea | Retrospective, single centre | 227 (144/83) | 100/100 | Doublet | 5%-10% received bevacizumab; 5%-10% received EGFR monoclonal antibody |
| Galizia et al[25] (2008) | 1995-2005 | Italy | Retrospective, single centre | 65 (42/23) | 100/100 | Singlet | Nil |
| Benoist et al[26] (2005) | 1997-2002 | France | Retrospective, case matched, single centre | 59 (32/27) | 94/100 | Singlet | Nil |
| Michel et al[21] (2004) | 1996-1999 | France | Retrospective, single centre | 54 (31/23) | 97/100 | Doublet | Nil |
| Ruo et al[43] (2003) | 1996-1999 | United States | Retrospective, single centre | 230 (127/103) | ND/83 | Singlet | Nil |
| Scoggins et al[44] (1999) | 1985-1997 | United States | Retrospective, single centre | 89 (66/23) | ND/100 | Singlet | Nil |
| Single arm: Primary chemotherapy | n | % group receiving chemo | |||||
| Yun et al[23] (2014) | 2000-2011 | South Korea | Retrospective, single centre | 259 | 100 | Doublet | ND |
| McCahill et al[16] (2012) | 2006-2009 | United States | Prospective Phase 2 | 86 | 100 | Doublet | 100% received bevacizumab |
| Clements et al[45] (2009) | 2003-2006 | United Kingdom | Retrospective, single centre | 37 | 92 | Doublet | ND |
| Bajwa et al[27] (2009) | 1999-2005 | United Kingdom | Retrospective, single centre | 67 | 100 | Doublet | ND |
| Poultsides et al[24] (2009) | 2000-2006 | United States | Retrospective, single centre | 233 | 100 | Doublet | 48% received bevacizumab 1st line |
| Muratore et al[46] (2007) | 2000-2004 | Italy | Prospective, single centre | 35 | 100 | Doublet | Nil |
| Sarela et al[47] (2001) | 1997-2000 | United Kingdom | Retrospective and prospective, single centre | 24 | 87 | Singlet | Nil |
| Single arm: Primary tumour resection | n | % group receiving chemo | |||||
| Maeda et al[28] (2013) | 2001-2009 | Japan | Retrospective, single centre | 94 | 85 | Doublet | 33% received targeted agent |
| Matsuda et al[17] (2012) | 1998-2007 | Japan | Retrospective, single centre | 40 | 74 | Doublet | ND |
PTR: Primary tumour resection; PC: Primary chemotherapy; ND: Not documented.
All studies included patients with both colon or rectal cancers, except Boselli et al[15] and McCahill et al[16] who excluded patients with rectal malignancies. All patients in the Matsuda et al[17] study had peritoneal metastases from a colorectal primary at diagnosis.
The vast majority of studies were single institution, retrospective reviews. The median age of patients ranged from 52-73. The proportion of males ranged from 50%-65%. The majority used modern 1st line chemotherapy regimes (fluoropyrimidine based doublet with oxaliplatin or irinotecan), though 5 studies conducted prior to the routine use of these agents used single agent fluoropyrimidine (5-fluorouracil) only, and in 1 study this data was missing. Five studies documented use of bevacizumab, though in many this data was missing, and only one study quoted specific use of EGFR monoclonal antibodies.
Outcomes
Overall survival: Median overall survival (Table 2) was compared in 11 studies. In the majority of studies, in acknowledgement of the risk of selection bias and confounding in retrospective studies, an attempt was made to provide adjusted survival data. This was presented as adjusted hazard ratios, or using matched patient cohorts.
Table 2.
Overall survival
| Ref. |
Unadjusted median OS (mo) |
Adjusted survival outcomes: Is PTR superior? | ||
| PTR | PC | P value | ||
| Galizia et al[25] (2008) | 15 | 12 | P = 0.03 | Yes (HR for death PC = 3.91, 95%CI: 2.83-4.99, P = 0.01) |
| Ahmed et al[42] (2014) Subgroup | 15 | 8 | P < 0.01 | Yes (analysis not shown) |
| Ruo et al[43] (2003) | 16 | 9 | P < 0.001 | No adjusted survival data |
| Yun et al[18] (2014) Matched cohort | 17 | 14 | P = NS | No (HR for death PC = 1.16, 95%CI: 0.89-1.52, P = 0.27) |
| Matsumoto et al[19] (2014) | 24 | 23 | P = NS | No (HR for death PTR = 0.72, 95%CI: 0.42-1.25, P = NS) |
| Seo et al[20] (2010) | 22 | 14 | P = NS | No (HR for death PC = 1.73, 95%CI: 0.94-3.16, P = 0.07) |
| Benoist et al[26] (2005) Matched cohort | 23 | 22 | P = NS | No (HR not reported, P = 0.753) |
| Cetin et al[22] (2013) | 23 | 17 | P = NS | No adjusted survival data |
| Michel et al[21] (2004) | 21 | 14 | P = NS | No adjusted survival data |
| Scoggins et al[44] (1999) | 14 | 17 | P = NS | No adjusted survival data |
| Boselli et al[15] (2013) | 4 | 5 | P = NS | No (HR for death PTR = 2.1, 95%CI: 1.06-4.5, P = 0.03) |
NS: Not significant (P > 0.05); PTR: Primary tumour resection; PC: Primary chemotherapy; HR: Hazard ratio.
In 3 studies, there was a statistically significant improvement in median overall survival in the PTR group. In 2 of these studies, this difference remained significant after adjustments, and in the third no attempt was made to calculate such adjustments. The magnitude of the unadjusted median overall survival benefit in these studies ranged from 3-7 mo.
In 7 studies, there was no statistically significant improvement in overall survival in the PTR group (and in 4 of these studies adjusted outcomes measures were used). However, in 3 of these studies (Yun et al[18]; Matsumoto et al[19]; and Seo et al[20]), there was a definite trend to an overall survival advantage with PTR that didn’t quite meet statistical significance. In 2 further studies[21,22], an unadjusted improvement in median overall survival in the PTR group of 7 and 6 mo respectively did not meet statistical significance, likely due to small sample sizes.
Only 1 study (Boselli et al[15]) suggested a survival disadvantage with PTR, but this study was an outlier (see Discussion below).
Primary tumour related complications: Sixteen studies looked at the rate of development of primary tumour related complications requiring intervention in patients undergoing PC (Table 3). This varied from 3.5% to 40%. The mean time to onset of complications ranged from 3-11 mo. The predominant complication was obstruction, with very low reported rates of bleeding, perforation and pain. Interventions to manage obstruction included both surgical (resection, de-functioning stoma or bypass procedures) and non-surgical (e.g., endoscopic stenting, radiotherapy, etc.). For the majority of these procedures, the authors commented that they were well tolerated and the patient was able to proceed with ongoing systemic treatment subsequently.
Table 3.
Primary tumour related complications in patients undergoing primary chemotherapy
| Ref. | % of patients requiring intervention for primary tumour related complications | Most common complication | Comment |
| Yun et al[18] (2014) | 3% | Obstruction > perforation | Mean onset of complications = 8 mo |
| Cetin et al[22] (2013) | 4% | Obstruction > rectovesical fistula | - |
| Muratore et al[46] (2007) | 6% | Obstruction > haemorrhage | - |
| Clements et al[45] (2009) | 8% | All obstruction | - |
| Scoggins et al[44] (1999) | 9% | All obstruction | Mean onset of complications = 3 mo |
| Poultsides et al[24] (2009) | 11% | Obstruction > perforation > pain | - |
| Seo et al[20] (2010) | 14% | Obstruction > bleeding | - |
| Benoist et al[26] (2005) | 15% | All obstruction | - |
| McCahill et al[16] (2012) | 16% | Obstruction > perforation, pain | Majority onset of complications < 12 mo |
| Michel et al[21] (2004) | 22% | All obstruction | Mean onset of complications = 4 mo |
| Yun et al[23] (2014) | 22% | Obstruction > perforation | Mean onset of complications = 7 mo |
| Matsumoto et al[19] (2014) | 26% | Majority obstruction | - |
| Ruo et al[43] (2003) | 29% | All obstruction | Majority onset of complications < 6 mo |
| Galizia et al[25] (2008) | 30% | Obstruction> perforation > haemorrhage | Mean onset of complication = 11 mo |
| Sarela et al[47] (2001) | 33% | Obstruction > pain > tenesmus | Mean onset of complication = 9 mo |
| Bajwa et al[27] (2009) | 40% | Obstruction > bleeding |
Three studies reviewed predictive variables for the development of complications requiring intervention. Matsumoto et al[19] identified inability to fully traverse the tumour at diagnostic colonoscopy as the only positive factor. For patients who subsequently developed obstruction, the mean time from diagnosis to onset was 2 mo in those with a non-traversable lesions vs 16 mo in those with a traversable lesion (P = 0.01). Yun et al[23] identified rectal tumours and tumours > 5 cm as positive predictive factors on multivariate analysis. Poultsides et al[24] did not find any positive correlation with reference to patient age, site of tumour, bevacizumab use, extent of metastatic disease, baseline CEA, Albumin, LDH or Alkaline phosphatase level.
Perioperative mortality and morbidity in PTR group: Peri-operative (30 d) mortality rates were reported in 12 studies (Table 4). In the vast majority, the rate was less than 2%. In the review by Boselli et al[15], there was a very high perioperative mortality rate (29%), but of note the PC group also had a high rate (19%), and the difference between the groups was not statistically significant.
Table 4.
Complications in patients undergoing primary tumour resection
| Ref. | Post-operative (30 d) mortality % |
Post-operative morbidity |
Requiring subsequent surgical intervention (%) | |
| % | Most common complication | |||
| Cetin et al[22] (2013) | 0 | ND | ND | 6% (all rectovesical fistula) |
| Benoist et al[26] (2005) | 0 | 19 | Wound infection, cardio-respiratory, intra-abdominal abscess, UTI | ND |
| Galizia et al[25] (2008) | 0 | 21 | All minor | 0% |
| Maeda et al[28] (2013) | 0 | 21 | Wound infection, ileus, anastomotic leak | ND |
| Michel et al[21] (2004) | 0 | ND | ND | ND |
| Seo et al[20] (2010) | 0 | 35 | Urine retention, wound complication, ileus. | 2% |
| Yun et al[18] (2014) | 1 | 10 | Ileus, wound infection, anastomotic leak | ND |
| Matsuda et al[17] (2012) | 2 | 15 | Wound infection, ileus | 11% |
| Ruo et al[43] (2003) | 2 | 21 | Wound infection, ileus, intra-abdominal infection | 3% |
| Matsumoto et al[19] (2014) | 2 | 20 | ND | ND |
| Scoggins et al[44] (1999) | 5 | 30 | Wound infection, UTI, sepsis | ND |
| Boselli et al[15] (2013) | 29 | 35 | Wound infection, UTI, pneumonia | ND |
ND: Not documented; UTI: Urinary tract infection;
With respect to morbidity, the most common post-operative complications were minor-wound infections, prolonged post-operative ileus, urinary infections/retention, and respiratory tract infections. Anastomotic leaks and intra-abdominal collections/sepsis were the most commonly reported major complications, and occurred in 0%-4% of patients in which specific complication rates were documented.
Impact on subsequent systemic therapy: Three studies looked at the median delay from diagnosis to commencement of chemotherapy, and predictably this was prolonged in the PTR group. In the Galizia et al[25] study, the interval was 35 d in the PTR group vs 8 d in the PC group (P < 0.01), in the Benoist et al[26] study 44 d vs 15 d respectively, and in the Seo et al[20] review 37 d vs 7 d respectively (P < 0.01).
The rates of significant (grade 3 or 4) chemotherapy related toxicities were also considered by the above authors, and no differences were identified between the groups in any study. Galizia et al[25] reported rates of 45% in PTR group vs 43% in PC group (P = 0.89), and Benoist et al[26] recorded 50% vs 37% respectively (P = 0.46). Seo et al[20] looked specifically at grade 3 or 4 gastro-intestinal toxicities, and the rates were similar between groups (10% vs 12 % respectively, P = 0.7).
Prognostic variables affecting overall survival: Eight studies looked at prognostic factors influencing overall survival in the whole cohort (PTR and PC groups combined). Table 5 summarises the variables found to be independently prognostic on multivariate analyses, and the hazard ratio for death (presence vs absence of factor) is documented where statistically significant.
Table 5.
Independent prognostic factors influencing overall survival on multivariate analysis, with hazard ratios or odds ratios for death
| Ref. | Age | Sex | ECOG PS ≥ 2 | Tumour location: Right colon | Tumour differentiation | T stage | N stage | M1b (vs M1a) | Presence of liver mets | Extent of hepatic involvement | Pre treatment CEA | Chemotherapy regime: Non use of Oxaliplatin/Irinotecan |
| Cetin et al[22] (2013) | a | a | a | |||||||||
| Yun et al[18] (2014)1 | a | a | a | a | a | a | HR 1.39 | HR 1.31 | a | |||
| Galizia et al[25] (2008) | a | a | HR 3.18 | a | a | a | a | HR 5.792 | a | |||
| Matsuda et al[17] (2013) | a | a | a | a | a | a | a | HR 2.57 | ||||
| Bajwa et al[27] (2009) | a | a | OR 2.61 | a | a | a | ||||||
| Maeda et al[28] (2013) | a | a | OR 2.73 | a | a | a | a | OR 1.66 | a | |||
| Seo et al[20] (2010) | a | a | a | a | HR 2.824 | a | HR 2.415 | a | HR 1.896 | |||
| Michel et al[21] (2004) | a |
a: Factor investigated by authors and found to be non-significant on multivariate analysis;
Unmatched cohort;
> 50% hepatic replacement (vs < 50% hepatic replacement);
ECOG PS ≥ 1 (vs 0);
High grade (vs low grade);
> 5 liver metastases (vs < 5);
Oxaliplatin use only. ECOG: Eastern Co-operative.
Age and sex were reviewed in most studies, and were not independent factors in any study. Performance status was examined in 4 studies, and was an independent factor in 2 of these, with hazard ratios for death of 2.7 and 3.2 for patients with an ECOG performance status ≥ 2 vs < 2. Bajwa et al[27] noted the presence of more than one primary tumour was a predictor for poorer overall survival in this cohort (OR for death 3.37, 95%CI: 1.21-9.3, P = 0.02).
The extent of hepatic parenchymal involvement by metastatic disease was a strong poor prognostic marker in 2 out of 4 reviews, with a hazard ratio for death of up to 5.8 for extensive disease vs limited disease. Metastatic dissemination to at least two distant sites (M1b stage) vs disease confined to one organ (M1a stage) conferred a worse prognoses in 2 out of 3 studies in which it was assessed, though the magnitude of the effect (hazard ratio) was low. In a review by Matsuda et al[17], for which the whole patient cohort had peritoneal carcinomatosis, the degree of peritoneal involvement (limited vs extensive) and the presence vs absence of ascites were not found to be significant prognostic factors.
In general, the location of the primary tumour (right colon vs left colon vs rectum) was not prognostic in this cohort. Only one review, by Bajwa et al[27] found tumours proximal to the splenic flexure conferred a worse prognosis than distal tumours (OR for death 2.61, P = 0.007). Tumour differentiation was again only prognostic in one study (Seo et al[20]), with “high grade” tumours (poorly differentiated, mucinous or signet ring histology) conferring a worse prognosis. T stage and N stage were not prognostic in this group with metastatic disease at diagnosis. Maeda et al[28] looked at two inflammation-based prognostic indices- the neutrophil to lymphocyte ratio and the Glasgow prognostic score (GPS), which scores patients based on their baseline level of C-reactive protein and Albumin at diagnosis (with points allocated for high C-reactive protein and hypoalbuminaemia). A neutrophil:lymphocyte ratio ≥ 3 (vs < 3) was associated with poorer survival on multivariate analysis (OR = 1.97, 95%CI: 1.74-3.39; P = 0.01), as was a GPS of 2 (vs 0-1) (OR = 1.95, 95%CI: 1.05-2.72; P = 0.03).
The use of doublet chemotherapy, with a 5-fluorouracil doublet (oxaliplatin or irinotecan) also improved survival in the 2 papers in which it was reviewed[17,20].
Only one study reviewed prognostic factors in subgroups (site of metastases) specific to the primary treatment modality (PTR vs PC). Yun et al[18] reported that, in their unmatched cohort, patients with liver, lung and peritoneal metastases all had improved survival in the PTR arm in comparison to the PC arm.
DISCUSSION
The decision regarding resection of an asymptomatic primary tumour, in a patient with a good performance status, is complex. For many, the key question is that of a survival advantage. The above summary suggests that both PTR and PC survival outcomes are equivalent, with a trend towards an overall survival advantage with PTR. In this cohort, PTR is relatively safe with most morbidity being minor and transient, and the vast majority of patients being able to proceed with systemic chemotherapy, with a mean delay of 5-7 wk post surgery. For the PC group, the most common complication is obstruction, with a median rate of occurrence of approximately 20%.
This review is novel because it looks specifically at asymptomatic patients receiving systemic chemotherapy in both arms, by default excluding those with poorer performance status. It is this cohort in whom the decision regarding PTR vs PC is the most complex for the multidisciplinary team. This review provides a current overview, including many recently published studies, with data collection in the modern chemotherapy era. Many previously published reviews of asymptomatic patients have included some studies with symptomatic primary tumours[29,30], or included trials with data mainly collected prior to 2005[31,32], when the therapeutic landscape was very different.
The trend to a survival advantage complements and parallels several studies looking at the general population (combined symptomatic and asymptomatic primary tumours at diagnosis). In a recent large meta-analysis of 15 studies involving 12416 patients by Ahmed et al[33], the median overall survival was 4 mo longer in patients undergoing PTR vs PC, and 6 mo longer in a subgroup receiving second and third generation chemotherapy. In a large cancer registry review by Tsang et al[8] of 11706 patients, all receiving chemotherapy, there was a 4 mo improvement in median overall survival in those undergoing PTR vs those declining it. Similarly, in a recent SEER database cohort review[34], using stratified propensity-score methods, there was a significantly improved overall and cancer-specific survival in patients undergoing PTR (adjusted HR of death = 0.40, 95%CI: 0.39-0.42; P < 0.001). However, the power of the model is limited by the prognostic variables available in the SEER database, which don’t include details regarding tumour burden and patient performance status, and thus selection bias is still a major limitation, though the magnitude of the benefit is hypothesis-generating.
However, the four papers with the most recent data in this review did not show an overall survival advantage with PTR. In an era where median overall survival in stage IV disease in recent trials is approaching 30 mo, it is possible that improved response rates may be enough to control asymptomatic primary lesions. In a small prospective trial of 16 patients by Karoui et al[12], 69% of primary colonic tumours achieved major histological tumour regression after neoadjuvant chemotherapy with FOLFOX or FOLFIRI chemotherapy. However, when comparing the histological response of the primary tumour compared with liver metastases, other small series have suggested that this may be poorer in the former[35], and this requires further investigation.
The main criticism of the current literature is the poor quality of evidence, with the vast majority of studies being retrospective, non-randomised single institution reviews, with their inherent risk of selection bias between the groups, and confounding. However, most reviews did attempt to control for these. Small sample sizes were another common limitation, and it is likely that many studies were underpowered to translate clinically significant improvements in overall survival into statistically significant results. Older meta-analyses including some of the trials in this review have suggested a survival advantage for PTR. Many included studies can also be criticized for their missing data in respect to accurate documentation of specific chemotherapy regimes or targeted agent used, which is a critical factor in the equation.
One review with anomalous results was Boselli et al[15], with very high surgical mortality and morbidity rates, and very low overall survival in both arms (4-5 mo). This was a small (n = 48), single institution review, with only 17 patients in the PTR group. The mean age of patients was older than other reviews (72), and significantly this cohort had a high proportion of patients with extensive hepatic metastatic disease (47% of PTR group and 58% of PC group has > 50% of liver parenchyma replaced by tumour) and a high proportion with documented hepatic failure (Childs Pugh B score 35% vs 55% respectively). All post-operative deaths were attributed to hepato-renal failure and heart failure. Given the significantly disproportionate results, reflecting a patient group skewed towards very extensive metastatic disease, the validity of this study is in question.
No quality of life data exists in the literature for this patient population. In the palliative setting, patient reported outcomes, both global quality of life (including functional outcomes) plus symptomatic scores are essential. Treatment options need to be evaluated with respect to their impact on symptoms which can significantly impair patients’ quality of life, such as pain, diarrhoea, tenesmus, faecal incontinence, etc. It is very likely that symptomatic local complications, particularly in rectal cancer patients, were under-reported in the included studies, given their retrospective nature. Pain from locally advanced rectal cancer can be an extremely debilitating complication, and other local complications can significantly impede social, emotional and physical functioning. Future studies should focus on global and symptomatic quality of life outcomes, and indeed most currently recruiting RCTs do have these as a secondary endpoint.
Most studies in this review failed to differentiate between colonic and rectal tumours. Anatomical restriction due to complex invasion patterns (e.g., to pelvic bones, genitourinary organs, major blood vessels and nerves) can make PTR more complicated or infeasible in rectal cancers. The use of up-front radiotherapy for “local control” was also poorly reported in the studies, and thus it is impossible to tease out the potential benefits of this as an alternative definitive primary therapy for rectal cancers. In addition, the cancers have different clinical trajectories. Given the above arguments, future trials should separate colon and rectal tumours as different entities, and look specifically at adjuvant or high dose palliative radiotherapy upfront, and the subsequent outcomes.
“Obstruction” was by far the most common delayed complication in the PC group. It must be remembered that in a proportion of patients presenting with obstructive symptoms, this may be due to peritoneal disease or adhesions secondary to surgeries, and therefore PTR is not a guarantee for prevention of such complications, and may contribute to such. Studies should compare complication rates in both arms, and only a minority of the papers did. The clinically relevant questions regarding the efficacy and morbidity of interventions to manage obstruction have not been covered in this review. Surgical therapeutic options include diverting stoma, internal bypass and palliative resection, and local therapies include laser coagulation and radiotherapy in rectal cancers. Many patients are now managed endoscopically with self-expanding metal stents (SEMS), and their rate of use has been increasing since their introduction in the 1990s. A recent meta-analysis of 88 trials involving patients using SEMS[36] reported a clinical success rate of 92%, with a median rate of re-intervention (required for stent blockage, migration, failure or perforation) of 20%. The efficacy and risks of each intervention are essential to relay to the patient if obstructive or other complications develop.
Many currently accepted prognostic variables in colorectal cancer reflect predictive markers for the development of metastatic disease (e.g., T stage, N stage, etc.). However, once patients have metastatic, incurable disease, the most relevant prognostic markers reflect the burden of disease and the patient’s overall performance status, and this is reflected by the findings above, with hepatic tumour burden, multiple sites of metastatic disease and poor ECOG performance status the most relevant indices. A criticism of most of the included papers is that they looked at prognostic factors for the whole cohort of patients, and did not differentiate between the treatment arms to assess for interaction. This data has been reported in the literature for the combined asymptomatic and symptomatic cohort. For example, in their pooled retrospective analysis of 4 first line chemotherapy trials, Faron et al[6] identified a significant interaction between PTR and the location of the primary tumour - the OS benefit of PTR being greater in rectal tumours than colon tumours. Tsang et al[8] used subgroup analyses based on tumour location and found that PTR conferred a significant survival advantage in both colon (OS HR = 0.39; 95%CI: 0.37-0.42; P < 0.0001) and rectal primaries (OS HR = 0.46; 95%CI: 0.43-0.50; P < 0.0001). The same authors analysed subgroups based on age, and in patients aged greater than 70 years, the survival benefit of PTR also persisted. Gresham et al[37] performed subgroup analyses based on extent of metastases, and found that the effect of PTR on OS was not modified by this. Most recently, Ishihara et al[38] used propensity score analysis to confirm a cancer-specific mortality benefit of PTR irrespective of number of organs involved in metastatic disease, and for locally advanced disease.
In tandem with all spheres of oncology, the decision regarding PTR vs PC needs to be individualised. More specific prognostic and predictive markers, to identify who may benefit from each strategy, are required. It is being increasingly appreciated that somatic mutation status is not only predictive of response to therapy, but also probably prognostic. In a retrospective review of 188 patients with colorectal cancer[39], those with KRAS mutations were found to have poorer outcomes, with a disease-specific survival of 2.6 years in KRAS mutant patients vs 4.8 years in wild type patients (P = 0.0003). Further work in this field is greatly anticipated.
There is no currently published randomised trial data because previously designed trials (including the ISAAC trial, ClinicalTrials.gov number NCT01086618) failed to recruit sufficiently. One reason for this may be the entrenched beliefs of clinicians, with a disparity between oncologists and surgeons. A recent survey of attitudes of clinicians showed that medical oncologists were more likely to prefer PC if a patient had an asymptomatic sigmoid or caecal lesion, whereas surgeons (colorectal and general) preferred a primary surgical approach[40]. Indeed, over the past two decades there has been a trend towards non operative management in Stage IV colorectal cancer, and the annual rate of PTR has decreased from 74.5% in 1988 to 57.4% in 2010 (P < 0.001)[41]. As the surgeon is usually the initial specialist for these patients, these beliefs may hinder recruitment to such trials. However, it is imperative that the currently recruiting RCTs do accrue enough patients to further clarify this grey area, and provide clarification on the suggestion of a survival advantage.
The above review advocates that both PTR followed by systemic chemotherapy and PC are appropriate up front treatment options in patients with asymptomatic primary lesions. There is a trend for a survival advantage in PTR, though the results of currently recruiting randomised trials and meta-analyses including recent trials are paramount to clarify this in the modern era. For those undergoing PTR, multiple studies confirm this is relatively safe and most patients can proceed to systemic treatment uneventfully. Patients with a higher burden of disease, particularly liver metastases, have poorer prognosis overall, though it remains to be clarified whether their primary mode of treatment modulates this, and the relevance of subgroups based on site, extent of disease and patient characteristics. Better validated prognostic tools are required to individualize patient management in this grey area.
COMMENTS
Background
The optimal management of the asymptomatic primary tumour in patients presenting with stage IV colorectal cancer is contentious, with no published randomised control trial data currently available.
Research frontiers
Previously published registry data and meta-analyses of retrospective small trials have suggested a possible survival advantage of primary tumour resection (PTR) in the general stage IV patient population. However, these have included patients with poor performance status unsuitable for aggressive therapy, and selection bias is a major limitation. In contrast, there has been a trend towards a reduction in the rates of primary tumour resection (PTR) in patients presenting with incurable disease. This discrepancy has peaked interest in the debate, and multiple randomised controlled trials are currently recruiting. In parallel with the trend for personalized oncology, identification of prognostic markers in this cohort and predictive markers for benefit or detriment of both management strategies are essential and currently lacking.
Innovations and breakthroughs
This review article is novel in its focus specifically on patients with good performance status presenting with asymptomatic lesions, who comprise the most complex clinical management challenge. Although most included papers suggest equivalent survival, the review identifies a trend to a survival advantage with PTR which is hampered by the methodological limitations of the included small studies. The review also highlights the relative safety of surgery in this cohort, and the minimal impact on subsequent systemic therapy. It also identifies the rate and nature of complications arising in patients undergoing non-surgical management and reports on what factors predict the development of such complications.
Applications
This review further informs members of the multidisciplinary team managing patients with incurable stage IV colorectal cancer, aiding their decision making based on the best available evidence to date.
Terminology
PTR: Primary tumour resection; PC: Primary chemotherapy.
Peer-review
In this review paper, Wilkinson et al evaluated management of asmptomatic primary tumours in stage IV colorectal cancer, referring previous studies. This is a carefully done study and the findings are of considerable interest.
Footnotes
Conflict-of-interest statement: No conflicts of interest are declared by the authors.
Data sharing statement: All data referred to in this review is taken directly from the outcome data from the original cited papers. No additional data are available.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: April 28, 2015
First decision: September 8, 2015
Article in press: November 4, 2015
P- Reviewer: Giannopoulos GA, Muguruma N, Rutegard J S- Editor: Ji FF L- Editor: A E- Editor: Wu HL
References
- 1.Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359–E386. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- 2.‘t Lam-Boer J, Mol L, Verhoef C, de Haan AF, Yilmaz M, Punt CJ, de Wilt JH, Koopman M. The CAIRO4 study: the role of surgery of the primary tumour with few or absent symptoms in patients with synchronous unresectable metastases of colorectal cancer--a randomized phase III study of the Dutch Colorectal Cancer Group (DCCG) BMC Cancer. 2014;14:741. doi: 10.1186/1471-2407-14-741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rahbari NN, Lordick F, Fink C, Bork U, Stange A, Jäger D, Luntz SP, Englert S, Rossion I, Koch M, et al. Resection of the primary tumour versus no resection prior to systemic therapy in patients with colon cancer and synchronous unresectable metastases (UICC stage IV): SYNCHRONOUS--a randomised controlled multicentre trial (ISRCTN30964555) BMC Cancer. 2012;12:142. doi: 10.1186/1471-2407-12-142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cotte E, Villeneuve L, Passot G, Boschetti G, Bin-Dorel S, Francois Y, Glehen O. GRECCAR 8: impact on survival of the primary tumor resection in rectal cancer with unresectable synchronous metastasis: a randomized multicentre study. BMC Cancer. 2015;15:47. doi: 10.1186/s12885-015-1060-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ferrand F, Malka D, Bourredjem A, Allonier C, Bouché O, Louafi S, Boige V, Mousseau M, Raoul JL, Bedenne L, et al. Impact of primary tumour resection on survival of patients with colorectal cancer and synchronous metastases treated by chemotherapy: results from the multicenter, randomised trial Fédération Francophone de Cancérologie Digestive 9601. Eur J Cancer. 2013;49:90–97. doi: 10.1016/j.ejca.2012.07.006. [DOI] [PubMed] [Google Scholar]
- 6.Faron M, Pignon JP, Malka D, Bourredjem A, Douillard JY, Adenis A, Elias D, Bouché O, Ducreux M. Is primary tumour resection associated with survival improvement in patients with colorectal cancer and unresectable synchronous metastases? A pooled analysis of individual data from four randomised trials. Eur J Cancer. 2015;51:166–176. doi: 10.1016/j.ejca.2014.10.023. [DOI] [PubMed] [Google Scholar]
- 7.Clancy C, Burke JP, Barry M, Kalady MF, Calvin Coffey J. A meta-analysis to determine the effect of primary tumor resection for stage IV colorectal cancer with unresectable metastases on patient survival. Ann Surg Oncol. 2014;21:3900–3908. doi: 10.1245/s10434-014-3805-4. [DOI] [PubMed] [Google Scholar]
- 8.Tsang WY, Ziogas A, Lin BS, Seery TE, Karnes W, Stamos MJ, Zell JA. Role of primary tumor resection among chemotherapy-treated patients with synchronous stage IV colorectal cancer: a survival analysis. J Gastrointest Surg. 2014;18:592–598. doi: 10.1007/s11605-013-2421-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.van der Burg ME, van Lent M, Buyse M, Kobierska A, Colombo N, Favalli G, Lacave AJ, Nardi M, Renard J, Pecorelli S. The effect of debulking surgery after induction chemotherapy on the prognosis in advanced epithelial ovarian cancer. Gynecological Cancer Cooperative Group of the European Organization for Research and Treatment of Cancer. N Engl J Med. 1995;332:629–634. doi: 10.1056/NEJM199503093321002. [DOI] [PubMed] [Google Scholar]
- 10.Flanigan RC, Salmon SE, Blumenstein BA, Bearman SI, Roy V, McGrath PC, Caton JR, Munshi N, Crawford ED. Nephrectomy followed by interferon alfa-2b compared with interferon alfa-2b alone for metastatic renal-cell cancer. N Engl J Med. 2001;345:1655–1659. doi: 10.1056/NEJMoa003013. [DOI] [PubMed] [Google Scholar]
- 11.Evans MD, Escofet X, Karandikar SS, Stamatakis JD. Outcomes of resection and non-resection strategies in management of patients with advanced colorectal cancer. World J Surg Oncol. 2009;7:28. doi: 10.1186/1477-7819-7-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Karoui M, Koubaa W, Delbaldo C, Charachon A, Laurent A, Piedbois P, Cherqui D, Tran Van Nhieu J. Chemotherapy has also an effect on primary tumor in colon carcinoma. Ann Surg Oncol. 2008;15:3440–3446. doi: 10.1245/s10434-008-0167-9. [DOI] [PubMed] [Google Scholar]
- 13.Cirocchi R, Trastulli S, Abraha I, Vettoretto N, Boselli C, Montedori A, Parisi A, Noya G, Platell C. Non-resection versus resection for an asymptomatic primary tumour in patients with unresectable stage IV colorectal cancer. Cochrane Database Syst Rev. 2012;8:CD008997. doi: 10.1002/14651858.CD008997.pub2. [DOI] [PubMed] [Google Scholar]
- 14.Benson AB, Bekaii-Saab T, Chan E, Chen YJ, Choti MA, Cooper HS, Engstrom PF, Enzinger PC, Fakih MG, Fenton MJ, et al. Metastatic colon cancer, version 3.2013: featured updates to the NCCN Guidelines. J Natl Compr Canc Netw. 2013;11:141–152; quiz 152. doi: 10.6004/jnccn.2013.0022. [DOI] [PubMed] [Google Scholar]
- 15.Boselli C, Renzi C, Gemini A, Castellani E, Trastulli S, Desiderio J, Corsi A, Barberini F, Cirocchi R, Santoro A, et al. Surgery in asymptomatic patients with colorectal cancer and unresectable liver metastases: the authors’ experience. Onco Targets Ther. 2013;6:267–272. doi: 10.2147/OTT.S39448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McCahill LE, Yothers G, Sharif S, Petrelli NJ, Lai LL, Bechar N, Giguere JK, Dakhil SR, Fehrenbacher L, Lopa SH, et al. Primary mFOLFOX6 plus bevacizumab without resection of the primary tumor for patients presenting with surgically unresectable metastatic colon cancer and an intact asymptomatic colon cancer: definitive analysis of NSABP trial C-10. J Clin Oncol. 2012;30:3223–3228. doi: 10.1200/JCO.2012.42.4044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Matsuda K, Hotta T, Takifuji K, Yokoyama S, Oku Y, Hashimoto T, Iwamoto H, Yamaue H. Clinical outcome of up-front surgery in patients with asymptomatic, incurable synchronous peritoneal carcinomatosis. Surg Today. 2013;43:984–989. doi: 10.1007/s00595-012-0348-9. [DOI] [PubMed] [Google Scholar]
- 18.Yun JA, Huh JW, Park YA, Cho YB, Yun SH, Kim HC, Lee WY, Chun HK. The role of palliative resection for asymptomatic primary tumor in patients with unresectable stage IV colorectal cancer. Dis Colon Rectum. 2014;57:1049–1058. doi: 10.1097/DCR.0000000000000193. [DOI] [PubMed] [Google Scholar]
- 19.Matsumoto T, Hasegawa S, Matsumoto S, Horimatsu T, Okoshi K, Yamada M, Kawada K, Sakai Y. Overcoming the challenges of primary tumor management in patients with metastatic colorectal cancer unresectable for cure and an asymptomatic primary tumor. Dis Colon Rectum. 2014;57:679–686. doi: 10.1097/DCR.0000000000000025. [DOI] [PubMed] [Google Scholar]
- 20.Seo GJ, Park JW, Yoo SB, Kim SY, Choi HS, Chang HJ, Shin A, Jeong SY, Kim DY, Oh JH. Intestinal complications after palliative treatment for asymptomatic patients with unresectable stage IV colorectal cancer. J Surg Oncol. 2010;102:94–99. doi: 10.1002/jso.21577. [DOI] [PubMed] [Google Scholar]
- 21.Michel P, Roque I, Di Fiore F, Langlois S, Scotte M, Tenière P, Paillot B. Colorectal cancer with non-resectable synchronous metastases: should the primary tumor be resected? Gastroenterol Clin Biol. 2004;28:434–437. doi: 10.1016/s0399-8320(04)94952-4. [DOI] [PubMed] [Google Scholar]
- 22.Cetin B, Kaplan MA, Berk V, Tufan G, Benekli M, Isikdogan A, Ozkan M, Coskun U, Buyukberber S. Bevacizumab-containing chemotherapy is safe in patients with unresectable metastatic colorectal cancer and a synchronous asymptomatic primary tumor. Jpn J Clin Oncol. 2013;43:28–32. doi: 10.1093/jjco/hys175. [DOI] [PubMed] [Google Scholar]
- 23.Yun JA, Park Y, Huh JW, Cho YB, Yun SH, Kim HC, Lee WY, Chun HK. Risk factors for the requirement of surgical or endoscopic interventions during chemotherapy in patients with uncomplicated colorectal cancer and unresectable synchronous metastases. J Surg Oncol. 2014;110:839–844. doi: 10.1002/jso.23725. [DOI] [PubMed] [Google Scholar]
- 24.Poultsides GA, Servais EL, Saltz LB, Patil S, Kemeny NE, Guillem JG, Weiser M, Temple LK, Wong WD, Paty PB. Outcome of primary tumor in patients with synchronous stage IV colorectal cancer receiving combination chemotherapy without surgery as initial treatment. J Clin Oncol. 2009;27:3379–3384. doi: 10.1200/JCO.2008.20.9817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Galizia G, Lieto E, Orditura M, Castellano P, Imperatore V, Pinto M, Zamboli A. First-line chemotherapy vs bowel tumor resection plus chemotherapy for patients with unresectable synchronous colorectal hepatic metastases. Arch Surg. 2008;143:352–338; discussion 358. doi: 10.1001/archsurg.143.4.352. [DOI] [PubMed] [Google Scholar]
- 26.Benoist S, Pautrat K, Mitry E, Rougier P, Penna C, Nordlinger B. Treatment strategy for patients with colorectal cancer and synchronous irresectable liver metastases. Br J Surg. 2005;92:1155–1160. doi: 10.1002/bjs.5060. [DOI] [PubMed] [Google Scholar]
- 27.Bajwa A, Blunt N, Vyas S, Suliman I, Bridgewater J, Hochhauser D, Ledermann JA, O’Bichere A. Primary tumour resection and survival in the palliative management of metastatic colorectal cancer. Eur J Surg Oncol. 2009;35:164–167. doi: 10.1016/j.ejso.2008.06.005. [DOI] [PubMed] [Google Scholar]
- 28.Maeda K, Shibutani M, Otani H, Nagahara H, Sugano K, Ikeya T, Amano R, Kimura K, Sakurai K, Kubo N, et al. Prognostic value of preoperative inflammation-based prognostic scores in patients with stage IV colorectal cancer who undergo palliative resection of asymptomatic primary tumors. Anticancer Res. 2013;33:5567–5573. [PubMed] [Google Scholar]
- 29.Eisenberger A, Whelan RL, Neugut AI. Survival and symptomatic benefit from palliative primary tumor resection in patients with metastatic colorectal cancer: a review. Int J Colorectal Dis. 2008;23:559–568. doi: 10.1007/s00384-008-0456-6. [DOI] [PubMed] [Google Scholar]
- 30.Kim YW, Kim IY. The Role of Surgery for Asymptomatic Primary Tumors in Unresectable Stage IV Colorectal Cancer. Ann Coloproctol. 2013;29:44–54. doi: 10.3393/ac.2013.29.2.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Scheer MG, Sloots CE, van der Wilt GJ, Ruers TJ. Management of patients with asymptomatic colorectal cancer and synchronous irresectable metastases. Ann Oncol. 2008;19:1829–1835. doi: 10.1093/annonc/mdn398. [DOI] [PubMed] [Google Scholar]
- 32.Stillwell AP, Buettner PG, Ho YH. Meta-analysis of survival of patients with stage IV colorectal cancer managed with surgical resection versus chemotherapy alone. World J Surg. 2010;34:797–807. doi: 10.1007/s00268-009-0366-y. [DOI] [PubMed] [Google Scholar]
- 33.Ahmed S, Shahid RK, Leis A, Haider K, Kanthan S, Reeder B, Pahwa P. Should noncurative resection of the primary tumour be performed in patients with stage iv colorectal cancer? A systematic review and meta-analysis. Curr Oncol. 2013;20:e420–e441. doi: 10.3747/co.20.1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tarantino I, Warschkow R, Worni M, Cerny T, Ulrich A, Schmied BM, Güller U. Prognostic Relevance of Palliative Primary Tumor Removal in 37,793 Metastatic Colorectal Cancer Patients: A Population-Based, Propensity Score-Adjusted Trend Analysis. Ann Surg. 2015;262:112–120. doi: 10.1097/SLA.0000000000000860. [DOI] [PubMed] [Google Scholar]
- 35.Gervaz P, Rubbia-Brandt L, Andres A, Majno P, Roth A, Morel P, Mentha G. Neoadjuvant chemotherapy in patients with stage IV colorectal cancer: a comparison of histological response in liver metastases, primary tumors, and regional lymph nodes. Ann Surg Oncol. 2010;17:2714–2719. doi: 10.1245/s10434-010-1056-6. [DOI] [PubMed] [Google Scholar]
- 36.Watt AM, Faragher IG, Griffin TT, Rieger NA, Maddern GJ. Self-expanding metallic stents for relieving malignant colorectal obstruction: a systematic review. Ann Surg. 2007;246:24–30. doi: 10.1097/01.sla.0000261124.72687.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gresham G, Renouf DJ, Chan M, Kennecke HF, Lim HJ, Brown C, Cheung WY. Association between palliative resection of the primary tumor and overall survival in a population-based cohort of metastatic colorectal cancer patients. Ann Surg Oncol. 2014;21:3917–3923. doi: 10.1245/s10434-014-3797-0. [DOI] [PubMed] [Google Scholar]
- 38.Ishihara S, Nishikawa T, Tanaka T, Tanaka J, Kiyomatsu T, Kawai K, Hata K, Nozawa H, Kazama S, Yamaguchi H, et al. Benefit of primary tumor resection in stage IV colorectal cancer with unresectable metastasis: a multicenter retrospective study using a propensity score analysis. Int J Colorectal Dis. 2015;30:807–812. doi: 10.1007/s00384-015-2228-4. [DOI] [PubMed] [Google Scholar]
- 39.Nash GM, Gimbel M, Shia J, Nathanson DR, Ndubuisi MI, Zeng ZS, Kemeny N, Paty PB. KRAS mutation correlates with accelerated metastatic progression in patients with colorectal liver metastases. Ann Surg Oncol. 2010;17:572–578. doi: 10.1245/s10434-009-0605-3. [DOI] [PubMed] [Google Scholar]
- 40.Wong R, Wiley M, Christophi C, Tebbutt N. Clinicians’ attitudes towards management of metastatic colorectal adenocarcinoma. ANZ J Surg. 2008;78:454–460. doi: 10.1111/j.1445-2197.2008.04534.x. [DOI] [PubMed] [Google Scholar]
- 41.Hu CY, Bailey CE, You YN, Skibber JM, Rodriguez-Bigas MA, Feig BW, Chang GJ. Time trend analysis of primary tumor resection for stage IV colorectal cancer: less surgery, improved survival. JAMA Surg. 2015;150:245–251. doi: 10.1001/jamasurg.2014.2253. [DOI] [PubMed] [Google Scholar]
- 42.Ahmed S, Leis A, Fields A, Chandra-Kanthan S, Haider K, Alvi R, Reeder B, Pahwa P. Survival impact of surgical resection of primary tumor in patients with stage IV colorectal cancer: results from a large population-based cohort study. Cancer. 2014;120:683–691. doi: 10.1002/cncr.28464. [DOI] [PubMed] [Google Scholar]
- 43.Ruo L, Gougoutas C, Paty PB, Guillem JG, Cohen AM, Wong WD. Elective bowel resection for incurable stage IV colorectal cancer: prognostic variables for asymptomatic patients. J Am Coll Surg. 2003;196:722–728. doi: 10.1016/S1072-7515(03)00136-4. [DOI] [PubMed] [Google Scholar]
- 44.Scoggins CR, Meszoely IM, Blanke CD, Beauchamp RD, Leach SD. Nonoperative management of primary colorectal cancer in patients with stage IV disease. Ann Surg Oncol. 1999;6:651–657. doi: 10.1007/s10434-999-0651-x. [DOI] [PubMed] [Google Scholar]
- 45.Clements D, Dhruva Rao P, Ramanathan D, Adams R, Maughan TS, Davies MM. Management of the asymptomatic primary in the palliative treatment of metastatic colorectal cancer. Colorectal Dis. 2009;11:845–848. doi: 10.1111/j.1463-1318.2008.01695.x. [DOI] [PubMed] [Google Scholar]
- 46.Muratore A, Zorzi D, Bouzari H, Amisano M, Massucco P, Sperti E, Capussotti L. Asymptomatic colorectal cancer with un-resectable liver metastases: immediate colorectal resection or up-front systemic chemotherapy? Ann Surg Oncol. 2007;14:766–770. doi: 10.1245/s10434-006-9146-1. [DOI] [PubMed] [Google Scholar]
- 47.Sarela AI, Guthrie JA, Seymour MT, Ride E, Guillou PJ, O’Riordain DS. Non-operative management of the primary tumour in patients with incurable stage IV colorectal cancer. Br J Surg. 2001;88:1352–1356. doi: 10.1046/j.0007-1323.2001.01915.x. [DOI] [PubMed] [Google Scholar]


