Figure 3.
COL13A1 Is Localized at the Human NMJ and Mediates Clustering of AChRs
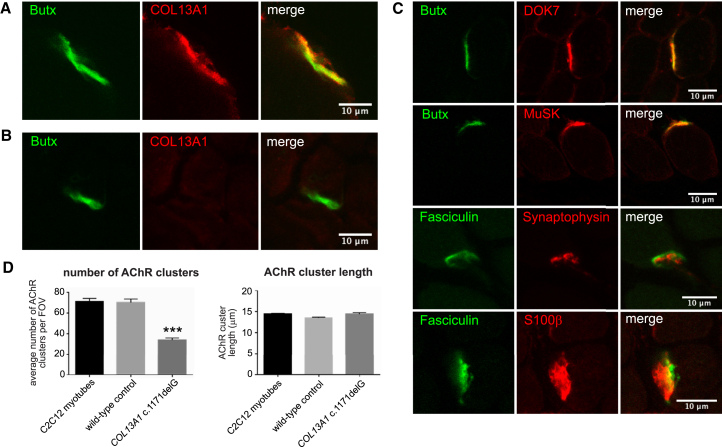
(A) In human muscle, COL13A1 (red) is enriched at endplate regions of NMJs marked by α-bungarotoxin (Butx; green). Scale bar represents 10 μm.
(B) Immunofluorescence labeling of quadriceps muscle from individual 1 (affected individual II:1 in family 1), who carries the homozygous frameshift mutation COL13A1 c.1171delG, which causes loss of COL13A1 accumulation (red) at an NMJ marked by α-bungarotoxin (Butx; green). Scale bar represents 10 μm.
(C) Normal expression and localization of markers for the NMJ (α-bungarotoxin [Butx]), the presynaptic terminal in NMJs (synaptophysin), terminal Schwann cells (S100β), the intersynaptic space (fasciculin), and postsynaptic proteins (DOK7 and MuSK) in individual 1. Scale bars represent 10 μm.
(D) AChR-cluster analysis of the COL13A1 c.1171delG variant. Bar graphs show that the frameshift mutation caused the number of AChR clusters in differentiated mutant myotubes to be statistically significantly lower than that in mock transfected (wild-type) or non-transfected C2C12 myotubes (left panel), but there was no significant effect on average cluster length (right panel). Statistical analyses used one-way ANOVA with Tukey’s multiple-comparison test (∗∗∗p < 0.001).