Abstract
The first example of a transition-metal-catalyzed, meta-selective C–H bromination procedure is reported. In the presence of catalytic [{Ru(p-cymene)Cl2}2], tetrabutylammonium tribromide can be used to functionalize the meta C–H bond of 2-phenylpyridine derivatives, thus affording difficult to access products which are highly predisposed to further derivatization. We demonstrate this utility with one-pot bromination/arylation and bromination/alkenylation procedures to deliver meta-arylated and meta-alkenylated products, respectively, in a single step.
Keywords: bromine, C–H activation, cross-coupling, regioselectivity, ruthenium
The field of catalytic C–H bond functionalization has grown significantly in recent years, thus offering new disconnections which can streamline synthetic routes and produce less waste.1 Several molecular architectures are now established for reliable C–H transformation, with arene C–H functionalization ortho to a directing group, by cyclometalation, being a prominent example.2 By contrast, meta functionalization is a more difficult reaction as the analogous cyclometalation processes are not at the chemists’ disposal. Given that stepwise meta functionalization is often challenging using classical arene chemistry, the development of new catalytic methods that address meta C–H functionality is of pressing importance.3 Several ground-breaking reaction systems have been developed to tackle this problem, principally in the areas of palladium and copper-catalyzed C–C bond formation,4–8 and iridium-catalyzed borylation.9 A third way of achieving meta functionalization has recently been described by the groups of Frost and Ackermann, where ruthenium catalysis is used for meta sulfonylation and alkylation, respectively.10 These reactions are thought to proceed by ortho ruthenation, thus affording an arylruthenium intermediate which exhibits a strong directing effect for functionalization at the C–H position para to the C–Ru bond.11 Addition of a suitable electrophile will thus result in overall meta substitution upon protonolysis of the C–Ru bond and completion of the catalytic cycle.
We were interested in exploring this concept in the context of meta bromination (Scheme 1). Aryl bromides are supremely versatile functional groups, with methods for C–H ortho bromination, and halogenation in general, undergoing extensive development in the C–H activation literature.12–17 However, meta bromination has yet to be described using transition-metal catalysis, and is restricted to very forcing reaction conditions in Friedel–Crafts bromination of electron-poor arenes (e.g., N–Br reagent in neat H2SO4 for bromination of nitrobenzene).18 A one-step meta-selective bromination, under mild reaction conditions, would open up a new pathway to valuable 1,3-bromo-functionalized arenes, which are currently prepared by tedious multistep routes. More generally, it would create a catalyst-controlled bromination system, where bromination of the same arene substrate could be directed to either the ortho- or meta-position depending upon the choice in catalyst.
scheme 1.
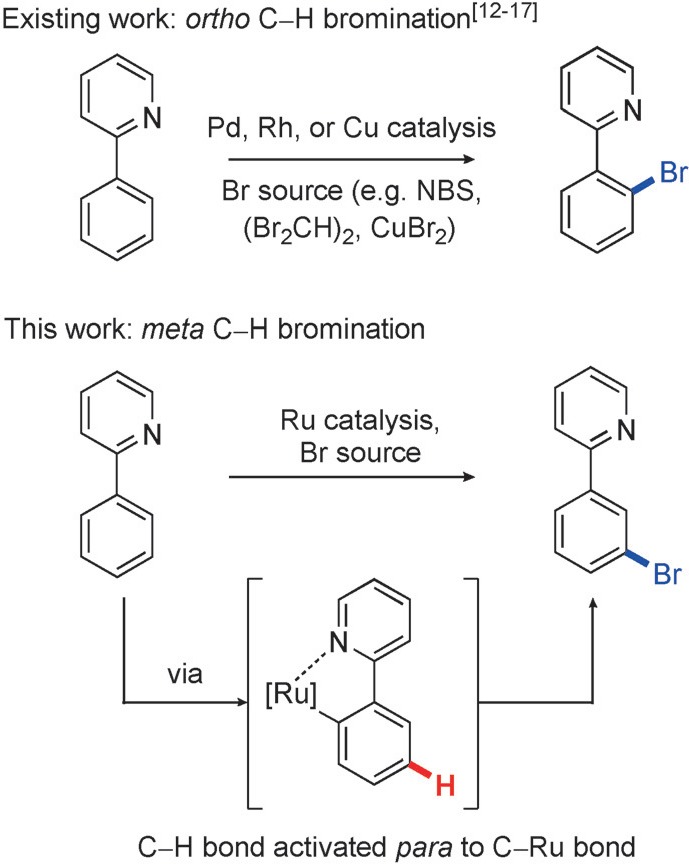
Transition-metal-catalyzed C–H bromination.
We began by screening electrophilic bromine sources in the presence of a base, catalytic [{Ru(p-cymene)Cl2}2], and 2-phenylpyridine (1 a), as the substrate. Initial results showed that NBS, bromine, and pyridinium tribromide gave minimal conversion to the desired meta-brominated product 2 a (Table 1, entries 1–5). The failure of pyridinium tribromide is notable (entry 5) as this reagent has been successfully used to stoichiometrically brominate organo-ruthenium complexes.11 Gratifyingly, we observed successful meta bromination on switching to tetrabutylammonium tribromide (TBATB) in 1,4-dioxane, with 2 a being formed with excellent conversion (entry 10). Use of a carboxylate additive in ruthenium catalyzed C–H activation chemistry has extensive precedent in work from the group of Ackermann,19 and acted in the current case to increase yields of the isolated products by 5–10 %. The reaction did not occur in the absence of ruthenium catalyst (entry 9) and in solvents other than 1,4-dioxane, no product was observed. Finally, the reaction was observed to be air-sensitive. In cases where the reaction was set up without rigorous removal of air, conversions were inconsistent but generally much lower.
Table 1.
Reaction development.
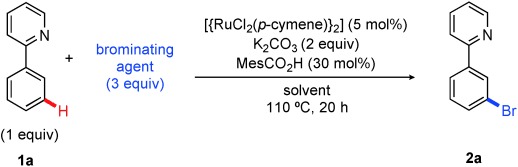
| Entry | Brominating agent | Solvent | 1 a/2 a[a] |
|---|---|---|---|
| 1 | NBS | acetonitrile | >99:1 |
| 2 | NBS | 1,4-dioxane | >95:5 |
| 3 | Br2 | acetonitrile | >99:1 |
| 4 | Br2 | 1,4-dioxane | >99:1 |
| 5 | pyridinium tribromide | 1,4-dioxane | >99:1 |
| 6 | TBATB | acetonitrile | >99:1 |
| 7 | TBATB | water | >99:1 |
| 8[b] | TBATB | 1,4-dioxane | 10:90 |
| 9[c] | TBATB | 1,4-dioxane | >99:1 |
| 10 | TBATB | 1,4-dioxane | 5:95 |
Ratio of 1 a/2 a is based on 1H NMR analysis of crude reaction mixtures after work-up.
Reaction carried out without MesCO2H additive.
Experiment carried out without ruthenium catalyst.
With the optimized reaction conditions in hand, we sought to explore the substrate scope (Scheme 2). We were pleased to find that both electron-donating (2 b–d) and electron-withdrawing groups (2 e–g) in the para-position of the aromatic ring were well tolerated, producing good to excellent yields of the bromide. In cases where the para-substituent possesses significant steric bulk the reaction still proceeds, but at a slower rate, thus resulting in a low yield after 20 hours (2 d). It should be noted that in low-yielding cases, the majority of the remaining material can be accounted for as starting the 2-phenylpyridine substrate. The reaction is remarkably selective for the monobrominated, rather than the dibrominated, product, despite using an excess of brominating agent. Over-bromination has been problematic in some previous examples of metal-catalyzed ortho bromination.13b, 15a The selectivity obtained in the meta bromination relative to other transition-metal-catalyzed bromination methods is exemplified by the reaction of benzo[h]quinoline to give the 7-brominated compound 2 j. This product could not be obtained selectively by using existing bromination methods,20 and it contains a new C–Br bond at a useful site for further modification. Functionalized benzoquinolines are used extensively as ligands in areas such as photoredox catalysis, metallo-supramolecular chemistry, and organic electronics, where methods for modifying the ligand structure are essential to fine-tune electronic properties.
scheme 2.
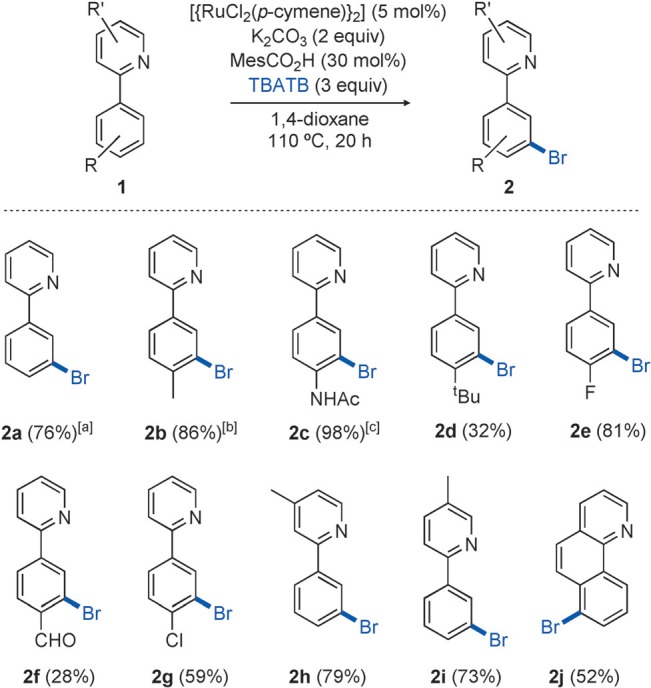
Substrate scope for meta bromination. Yields are those of isolated products. [a] Average of three runs. [b] Average of two runs. [c] Yield without ruthenium catalyst is 10 %. NBS=N-bromosuccinimide.
As with the sulfonylation system reported by Frost and co-workers,10a meta substitution on the phenyl ring is not tolerated. Ruthenation is presumably directed to the most sterically accessible ortho position, meaning that the pre-existing meta substituent is now blocking the site of bromination. Likewise, ortho substitution was not tolerated in phenylpyridine substrates. It is likely that this additional steric encumbrance prevents co-planarity of the phenyl–pyridine biaryl, thus disrupting the directed metalation and ensuing bromination. However, with substitution at other positions on the pyridine directing group, the reaction proceeds in excellent yield (2 h,i). Pleasingly, we were able to scale-up the reaction to a 5 mmol scale. By running the reaction for 65 hours, but with half the catalyst loading (2.5 mol %), the yield of isolated 2 a remained at 76 %, with 4 % of the ortho-brominated product also isolated.
To demonstrate the versatility of this methodology, we developed simple one-pot processes to further manipulate the newly installed bromide group in C–C bond-forming reactions (Scheme 3).21 We could meta-arylate by a one-pot bromination/Suzuki–Miyaura coupling: additional base was used in the first step, and after running the bromination for 20 hours, water, Pd(OAc)2 (3 mol %), PPh3 (6 mol %), and either a boronic acid or ester (3 equiv) were added and the reaction run for a further 15 hours. This one-pot meta-arylation procedure worked well for ortho-, meta-, and para- substituted boronic acids, and both electron-withdrawing and electron-donating substituents were tolerated (3 a–e). The reaction was extended to heteroaromatic boronic esters, with the use of N-Boc-pyrrole-2-boronic acid MIDA ester proving effective for the synthesis of 3 f in 64 % yield. A meta-alkenylation process was also possible: by simply adding Pd(OAc)2 (3 mol %) and three equivalents of a suitable alkene, post-bromination, and heating the reaction to 110 °C a one-pot bromination/Heck reaction proceeded. Yields of the alkenylated product over the two steps were good (4 a and 4 c), and the use of but-3-en-2-ol gave the alkylated ketone product 4 b in 55 % yield.
scheme 3.
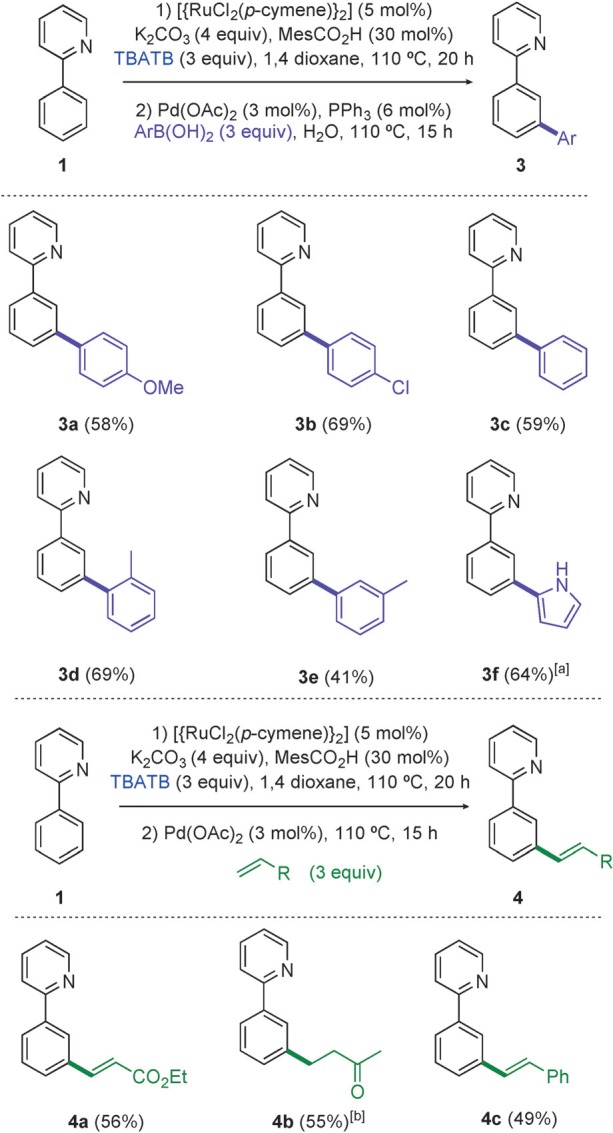
Substrate scope for one-pot transformations. Yields are those of isolated products. [a] N-Boc-pyrrole-2-boronic acid MIDA ester used as boronic acid starting material. [b] But-3-en-2-ol used as olefin starting material. MIDA=N-methyliminodiacetic acid.
Finally, we could successfully convert the pyridine directing group into the saturated heterocycle 5 (Scheme 4). Pyridine reduction is a versatile entry point into functionalized piperidines, which are heavily exploited scaffolds in medicinal chemistry. Here, treatment of 2 a with SmI2/H2O rapidly reduced the heteroarene,22 thus leaving the aryl bromide group intact for further manipulation.
scheme 4.
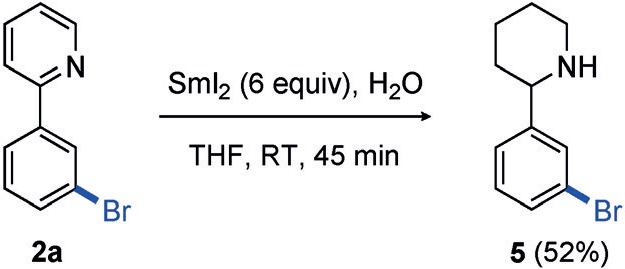
Reduction of pyridine directing group using SmI2. THF= tetrahydrofuran.
To conclude, we report the first example of transition-metal-catalyzed meta-selective bromination. The orthogonal selectivity exhibited by ruthenium relative to copper, palladium, and rhodium catalysis offers a catalyst-controlled route to compounds which may have been previously difficult to synthesize. Further, the reaction system is amenable to one-pot, telescoped processes which enable meta arylation and meta alkenylation. Further investigations into the scope of this chemistry are currently underway in our laboratory.
Acknowledgments
We thank the EPSRC for funding (PhD studentship to C.J.T. and Leadership Fellowship to M.F.G.). Dr. Scott Cockroft (University of Edinburgh) is thanked for helpful discussions. Dr. Nicolas Kern and Pauline Rabet (University of Manchester) are thanked for assistance with SmI2 and starting-material synthesis, respectively.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
miscellaneous_information
References
- 1.“C–H Activation”: Topics in Current Chemistry, Vol. 292 (Eds.: Yu J.-Q, Shi Z.), Berlin, Springer, 2010. [PubMed] [Google Scholar]
- 2.Reviews:
- 2a.Daugulis O, Do H-Q, Shabashov D. Acc. Chem. Res. 2009;42:1074–1086. doi: 10.1021/ar9000058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2b.Albrecht M. Chem. Rev. 2010;110:576–623. doi: 10.1021/cr900279a. [DOI] [PubMed] [Google Scholar]
- 2c.Colby DA, Bergman RG, Ellman JA. Chem. Rev. 2010;110:624–655. doi: 10.1021/cr900005n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2d.Engle KM, Mei T-S, Wasa M, Yu J-Q. Acc. Chem. Res. 2012;45:788–802. doi: 10.1021/ar200185g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2e.Neufeldt SR, Sanford MS. Acc. Chem. Res. 2012;45:936–946. doi: 10.1021/ar300014f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Reviews:
- 3a.Yang J. Org. Biomol. Chem. 2015;13:1930–1941. doi: 10.1039/c4ob02171a. [DOI] [PubMed] [Google Scholar]
- 3b.Juliá-Hernández F, Simonetti M, Larrosa I. Angew. Chem. Int. Ed. 2013;52:11458–11460. doi: 10.1002/anie.201306425. [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2013;125 [Google Scholar]
- 4a.Phipps RJ, Gaunt MJ. Science. 2009;323:1593–1597. doi: 10.1126/science.1169975. [DOI] [PubMed] [Google Scholar]
- 4b.Duong HA, Gilligan RE, Cooke ML, Phipps RJ, Gaunt MJ. Angew. Chem. Int. Ed. 2011;50:463–466. doi: 10.1002/anie.201004704. [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2011;123 [Google Scholar]
- 4c.Zhang Y-H, Shi B-F, Yu J-Q. J. Am. Chem. Soc. 2009;131:5072–5074. doi: 10.1021/ja900327e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5a.Leow D, Li G, Mei TS, Yu J-Q. Nature. 2012;486:518–522. doi: 10.1038/nature11158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5b.Dai H-X, Li G, Zhang X-G, Stepan AF, Yu J-Q. J. Am. Chem. Soc. 2013;135:7567–7571. doi: 10.1021/ja400659s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5c.Wan L, Dastbaravardeh N, Li G, Yu J-Q. J. Am. Chem. Soc. 2013;135:18056–18059. doi: 10.1021/ja410760f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5d.Lee S, Lee H, Tan KL. J. Am. Chem. Soc. 2013;135:18778–18781. doi: 10.1021/ja4107034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5e.Yang Y-F, Cheng G-J, Liu P, Leow D, Sun TY, Chen P, Zhang X, Yu JQ, Wu YD, Houk KN. J. Am. Chem. Soc. 2014;136:344–355. doi: 10.1021/ja410485g. [DOI] [PubMed] [Google Scholar]
- 5f.Tang R-Y, Li G, Yu J-Q. Nature. 2014;507:215–220. doi: 10.1038/nature12963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Luo J, Preciado S, Larrosa I. J. Am. Chem. Soc. 2014;136:4109–4112. doi: 10.1021/ja500457s. [DOI] [PubMed] [Google Scholar]
- 7a.Wang X-C, Gong W, Fang L-Z, Zhu R-Y, Li S, Engle KM, Yu J-Q. Nature. 2015;519:334–338. doi: 10.1038/nature14214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7b.Zhe D, Wang J, Dong G. J. Am. Chem. Soc. 2015;137:5887–5890. doi: 10.1021/jacs.5b02809. [DOI] [PubMed] [Google Scholar]
- 8.Zhang J, Liu Q, Liu X, Zhang S, Jiang P, Wang Y, Luo S, Li Y, Wang Q. Chem. Commun. 2015;51:1297–1300. doi: 10.1039/c4cc07997c. [DOI] [PubMed] [Google Scholar]
- 9a.Cho J-Y, Tse MK, Holmes D, Maleczka RE, Smith MR. Science. 2002;295:305–308. doi: 10.1126/science.1067074. [DOI] [PubMed] [Google Scholar]
- 9b.Ishiyama T, Takagi J, Ishida K, Miyaura N, Anastasi NR, Hartwig JF. J. Am. Chem. Soc. 2002;124:390–391. doi: 10.1021/ja0173019. [DOI] [PubMed] [Google Scholar]
- 10a.Saidi O, Marafie J, Ledger AEW, Liu PM, Mahon MF, Kociok-Kohn G, Whittlesey MK, Frost CG. J. Am. Chem. Soc. 2011;133:19298–19301. doi: 10.1021/ja208286b. [DOI] [PubMed] [Google Scholar]
- 10b.Reynolds WR, Liu PM, Kociok-Köhn G, Frost CG. Synlett. 2013:2687–2690. [Google Scholar]
- 10c.Hofmann N, Ackermann L. J. Am. Chem. Soc. 2013;135:5877–5884. doi: 10.1021/ja401466y. [DOI] [PubMed] [Google Scholar]
- 11.For studies on the functionalization of arylruthenium complexes, see:
- 11a.Clark AM, Rickard CEF, Roper WR, Wright LJ. Organometallics. 1999;18:2813–2820. [Google Scholar]
- 11b.Clark AM, Rickard CEF, Roper WR, Wright LJ. J. Organomet. Chem. 2000;598:262–275. [Google Scholar]
- 12.Seminal work:
- 12a.Fahey DR. J. Organomet. Chem. 1971;27:283–292. [Google Scholar]
- 12b.Carr K, Sutherland JK. Chem. Commun. 1984:1227–1228. [Google Scholar]
- 12c.Baldwin JE, Jones RH, Najera C, Yus M. Tetrahedron. 1985;41:699–711. [Google Scholar]
- 12d.Andrienko OS, Goncharov VS, Raida VS. Russ. J. Org. Chem. 1996;32:89–92. [Google Scholar]
- 13.Copper catalysis:
- 13a.Chen X, Hao X-S, Goodhue CE, Yu J-Q. J. Am. Chem. Soc. 2006;128:6790–6791. doi: 10.1021/ja061715q. [DOI] [PubMed] [Google Scholar]
- 13b.Wang W, Pan C, Chen F, Cheng J. Chem. Commun. 2011;47:3978–3980. doi: 10.1039/c0cc05557c. [DOI] [PubMed] [Google Scholar]
- 13c.Li B, Liu B, Shi B-F. Chem. Commun. 2015;51:5093–5096. doi: 10.1039/c5cc00531k. [DOI] [PubMed] [Google Scholar]
- 14.Palladium catalysis:
- 14a.Dick AR, Hull KL, Sanford MS. J. Am. Chem. Soc. 2004;126:2300–2301. doi: 10.1021/ja031543m. [DOI] [PubMed] [Google Scholar]
- 14b.Giri R, Chen X, Yu J-Q. Angew. Chem. Int. Ed. 2005;44:2112–2115. doi: 10.1002/anie.200462884. [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2005;117 [Google Scholar]
- 14c.Wan X, Ma Z, Li B, Zhang K, Cao S, Zhang S, Shi Z. J. Am. Chem. Soc. 2006;128:7416–7417. doi: 10.1021/ja060232j. [DOI] [PubMed] [Google Scholar]
- 14d.Kalyani D, Dick AR, Anani WQ, Sanford MS. Org. Lett. 2006;8:2523–2526. doi: 10.1021/ol060747f. [DOI] [PubMed] [Google Scholar]
- 14e.Whitfield SR, Sanford MS. J. Am. Chem. Soc. 2007;129:15142–15143. doi: 10.1021/ja077866q. [DOI] [PubMed] [Google Scholar]
- 14f.Whitfield SR, Sanford MS. Organometallics. 2008;27:1683–1689. [Google Scholar]
- 14g.Mei T-S, Giri R, Maugel N, Yu J-Q. Angew. Chem. Int. Ed. 2008;47:5215–5219. doi: 10.1002/anie.200705613. [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2008;120 [Google Scholar]
- 14h.Kakiuchi F, Kochi T, Mutsutani H, Kobayashi N, Urano S, Sato M, Nishiyama S, Tanabe T. J. Am. Chem. Soc. 2009;131:11310–11311. doi: 10.1021/ja9049228. [DOI] [PubMed] [Google Scholar]
- 14i.Zhao X, Dimitrijević E, Dong VM. J. Am. Chem. Soc. 2009;131:3466–3467. doi: 10.1021/ja900200g. [DOI] [PubMed] [Google Scholar]
- 14j.Song B, Zheng X, Mo J, Xu B. Adv. Synth. Catal. 2010;352:329–335. [Google Scholar]
- 14k.Dudnik AS, Chernyak N, Huang C, Gevorgyan V. Angew. Chem. Int. Ed. 2010;49:8729–8732. doi: 10.1002/anie.201004426. [DOI] [PMC free article] [PubMed] [Google Scholar]; Angew. Chem. 2010;122 [Google Scholar]
- 14l.Powers DC, Xiao DY, Geibel MAL, Ritter T. J. Am. Chem. Soc. 2010;132:14530–14536. doi: 10.1021/ja1054274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14m.Bedford RB, Haddow MF, Mitchell CJ, Webster RL. Angew. Chem. Int. Ed. 2011;50:5524–5527. doi: 10.1002/anie.201101606. [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2011;123 [Google Scholar]
- 14n.Dubost E, Fossey C, Cailly T, Rault S, Fabis F. J. Org. Chem. 2011;76:6414–6420. doi: 10.1021/jo200853j. [DOI] [PubMed] [Google Scholar]
- 14o.John A, Nicholas KM. J. Org. Chem. 2012;77:5600–5606. doi: 10.1021/jo300713h. [DOI] [PubMed] [Google Scholar]
- 14p.Wang X-C, Hu Y, Bonacorsi S, Hong Y, Burrell R, Yu J-Q. J. Am. Chem. Soc. 2013;135:10326–10329. doi: 10.1021/ja4055492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rhodium catalysis:
- 15a.Schröder N, Wencel-Delord J, Glorius F. J. Am. Chem. Soc. 2012;134:8298–8301. [Google Scholar]
- 15b.Qian G, Hong X, Liu B, Mao H, Xu B. Org. Lett. 2014;16:5294–5297. doi: 10.1021/ol502447w. [DOI] [PubMed] [Google Scholar]
- 15c.Hwang H, Kim J, Jeong J, Chang S. J. Am. Chem. Soc. 2014;136:10770–10776. doi: 10.1021/ja5053768. [DOI] [PubMed] [Google Scholar]
- 15d.Schroeder N, Lied F, Glorius F. J. Am. Chem. Soc. 2015;137:1448–1451. doi: 10.1021/jacs.5b00283. [DOI] [PubMed] [Google Scholar]
- 16.Cobalt catalysis: Yu D-G, Gensch T, de Azambuja F, Vasquez-Cespedes S, Glorius F.J. Am. Chem. Soc 201413617722–17725. [DOI] [PubMed] [Google Scholar]
- 17.Ruthenium catalysis: Wang L, Ackermann L.Chem. Commun 2014501083–1085. [DOI] [PubMed] [Google Scholar]
- 18a.Jacquesy J-C, Jouannetaud M-P, Makani S. Chem. Commun. 1980:110–111. [Google Scholar]
- 18b.de Almeida LS, Esteves PM, de Mattos MCS. Tetrahedron Lett. 2009;50:3001–3004. [Google Scholar]
- 19.Ackermann L. Acc. Chem. Res. 2014;47:281–295. doi: 10.1021/ar3002798. [DOI] [PubMed] [Google Scholar]
- 20.The 7-chloro analogue of 2 j was recently prepared in 13 % overall yield through a nitration/reduction/Sandmeyer sequence on benzo[h]quinolone. See: Furuya T, Benitez D, Tkatchouk E, Strom AE, Tang P, Goddard WA, Ritter T.J. Am. Chem. Soc 20101323793–3807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.For tandem meta arylation by borylation/Suzuki–Miyaura chemistry, see:
- 21a.Ishiyama T, Nobuta Y, Hartwig JF, Miyaura N. Chem. Commun. 2003:2924–2925. doi: 10.1039/b311103b. [DOI] [PubMed] [Google Scholar]
- 21b.Mkhalid IAI, Coventry DN, Albesa-Jove D, Batsanov AS, Howard JAK, Perutz RN, Marder TB. Angew. Chem. Int. Ed. 2006;45:489–491. doi: 10.1002/anie.200503047. [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2006;118 [Google Scholar]
- 21c.Paul S, Chotana GA, Holmes D, Reichle RC, Maleczka RE, Jr, Smith MR., III J. Am. Chem. Soc. 2006;128:15552–15553. doi: 10.1021/ja0631652. [DOI] [PubMed] [Google Scholar]
- 21d.Kikuchi T, Nobuta Y, Umeda J, Yamamoto Y, Ishiyama T, Miyaura N. Tetrahedron. 2008;64:4967–4971. [Google Scholar]
- 21e.Harrisson P, Morris J, Steel PG, Marder TB. Synlett. 2009:147–150. doi: 10.1021/ol901306m. [DOI] [PubMed] [Google Scholar]
- 21f.Beck EM, Hatley R, Gaunt MJ. Angew. Chem. Int. Ed. 2008;47:3004–3007. doi: 10.1002/anie.200705005. [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2008;120 [Google Scholar]
- 22a.Kamochi Y, Kudo T. Heterocycles. 1993;36:2383–2396. [Google Scholar]
- 22b.Szostak M, Spain M, Procter DJ. J. Org. Chem. 2014;79:2522–2537. doi: 10.1021/jo4028243. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
miscellaneous_information


