Fig. 2.
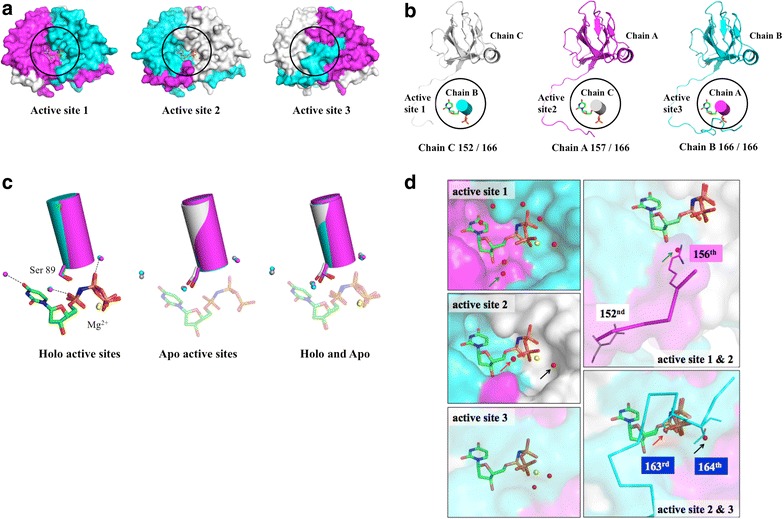
The structures of the apo and holo forms of Arabidopsis dUTPase. a Surface presentation of the dUTPase trimer. Circles indicate the active sites. Chains A, B, and C are colored in magenta, cyan, and gray, respectively. b The structure of each active site is composed of three subunits. Each diagram contains a ligand, an α-helix carrying Ser89 and Trp93, and a subunit that provides the C-terminal residues to the active site in a cartoon model. The lengths of the C-termini of the subunits are different. c Apo and holo common water molecules at the active site. Stick models represent the ligand and the ligand-interacting serine residue (S89). d Ligand interaction–replacement by water molecules and amino acids. The water molecules indicated by the arrow are initially associated with the ligand and later potentially replaced with C-terminal amino acids
