Figure 2.
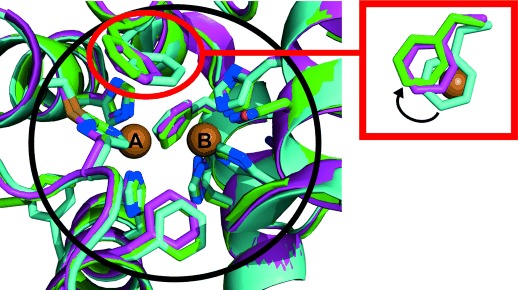
Superimposition of the active sites of jrTYR, ibCO, and vvCO. Proteins are shown in cartoon representation with the active-site residues illustrated as stick models and each structure colored differently: jrTYR in green, ibCO in cyan, and vvCO in purple (green/cyan/purple C, blue N, yellow S, red O). The big black circle delineates the area of the first shell of the active site (ca. 5.5 Å around each copper). The red circle with the associated inset indicates the different positions of the phenylalanine residues above CuA as seen from the top, with CuA (from jrTYR) drawn as a brown sphere. The phenylalanine of ibCO (cyan) covers CuA totally, whereas only partial CuA shielding can be observed for the phenylalanine of vvCO (purple). The position of the phenylalanine side chain of jrTYR (green) leads to no shielding. The black arrow in the inset indicates the shift from total CuA coverage (ibCO, cyan) to no coverage (jrTYR, green).
