Table 1.
Reaction development and optimization.
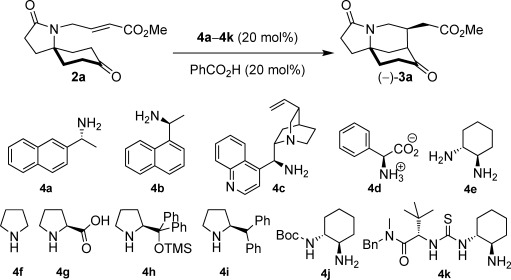
| Entry | Cat. | t | Yield[a] [%] | d.r.[b] | ee[c] [%] |
|---|---|---|---|---|---|
| 1 | 4 a | 5 days | 72 | >98:2 | 63 |
| 2 | 4 b | 8 days | 74 | >98:2 | 69[d] |
| 3 | 4 c | 7 days | 69 | >98:2 | 31 |
| 4 | 4 d | NR | – | – | – |
| 5 | 4 e | 24 h | 82 | >98:2 | 63 |
| 6 | 4 f–4 i | NR | – | – | – |
| 7 | 4 j | 22 h | 87 | >98:2 | 64 |
| 8 | 4 k | 26 h | 86 | >98:2 | 90 |
| 9[e] | 4 k | 25 h | 78 | >98:2 | 90 |
| 10[f] | 4 k | 96 h | 80 | >98:2 | 92 |
| 11[g] | 4 k | 48 h | 88 | >98:2 | 93 |
Yield of isolated product after flash column chromatography.
Diastereomeric ratios (d.r.) were determined by 1H NMR spectroscopy.
The ee values were determined by HPLC analysis on a chiral stationary phase.
(+)-3 a was obtained.
CHCl3 as the solvent.
4 k (5 mol %), benzoic acid (1.25 mol %), RT.
4 k (5 mol %), benzoic acid (1.25 mol %), 45 °C. See the Supporting Information for details. Bn=benzyl, Boc=tert-butyloxycarbonyl, NR=no reaction. TMS=trimethylsilyl.
