Table 3.
Scope of the ketone aldol/cyclization reaction with isocyanoacetates. 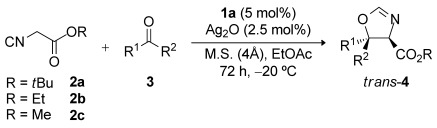
| Entry | 2 | 3 | R1 | R2 | 4 | Yield [%][a] | d.r.[b] | e.r.[c] |
|---|---|---|---|---|---|---|---|---|
| 1 | 2 a | 3 a | Ph | Me | 4 a | 84 | 94:6 | 94:6 |
| 2 | 2 a | 3 b | p-CH3OC6H4 | Me | 4 b | 73 | 92:8 | 94:6 |
| 3 | 2 a | 3 c | 5-Br-thiophen-2-yl | Me | 4 c | 78 | 88:12 | 93:7 |
| 4 | 2 a | 3 d | p-NO2C6H4 | Me | 4 d | 60 | 90:10 | 95:5 |
| 5 | 2 a | 3 e | p-BrC6H4 | Me | 4 e | 71 | 89:11 | 95:5 |
| 6 | 2 a | 3 f | p-CNC6H4 | Me | 4 f | 80 | 90:10 | 96:4 |
| 7 | 2 a | 3 g | 4-F,3-BrC6H3 | Me | 4 g | 83 | 85:15 | 89:11 |
| 8 | 2 a | 3 h | 3,5-(CF3)2C6H3 | Me | 4 h | 82 | 86:14 | 88:12 |
| 9 | 2 a | 3 i | 5-methylthiazol-2-yl | Me | 4 i | 55 | 91:9 | 93:7 |
| 10 | 2 a | 3 j | pyrazin-2-yl | Me | 4 j | 75 | 91:9 | 91:9 |
| 11 | 2 a | 3 k | Ph | Et | 4 k | 76 | 90:10 | 99:1 |
| 12 | 2 a | 3 l | p-MeC6H4 | Et | 4 l | 81 | 91:9 | 98:2 |
| 13 | 2 a | 3 m | p-FC6H4 | Et | 4 m | 83 | 88:12 | 99:1 |
| 14 | 2 a | 3 n | p-BrC6H4 | Et | 4 n | 73 | 90:10 | 99:1 |
| 15 | 2 a | 3 o | Ph | Pr | 4 o | 79 | 87:13 | 99:1 |
| 16 | 2 a | 3 p | 2-thienyl | Pr | 4 p | 81 | 84:16 | 96:4 |
| 17 | 2 a | 3 q | Ph | CH2iPr | 4 q | 75 | 96:4 | 97:3 |
| 18 | 2 b | 3 k | Ph | Et | 4 r | 81 | 90:10 | 99:1 |
| 19 | 2 b | 3 l | p-MeC6H4 | Et | 4 s | 82 | 91:9 | 98:2 |
| 20 | 2 c | 3 k | Ph | Et | 4 t | 77 | 91:9 | 99:1 |
Combined yield of both diastereomers after flash column chromatography.
Determined by 1HNMR analysis of the crude reaction mixture.
Determined by HPLC analysis on a chiral stationary phase.
