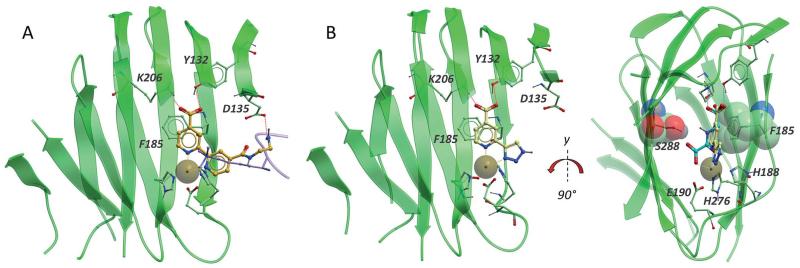
Fig. 2.
A. Overlay of a 2,2′-bipyridine inhibitor (orange sticks, from PDB ID: 3PDQ)23 and H3K9me3 peptide (lavender ribbon and sticks, from PDB ID: 2OQ6)32 in KDM4A (green ribbon and sticks, from PDB ID: 3PDQ). The inhibitor forms a H-bond to Y132 and two salt bridges to K206 and D135 (red dashed lines). The bipyridine motif coordinates the nickel atom (orange dashed lines to brown sphere) which replaces the catalytic iron in the crystal structure. The diaminoethane substituent extends into the peptide-binding pocket. B. Overlay of compound 14a (pale sticks, from PDB ID: 4URA) and NOG, 2 (cyan sticks, from PDB ID: 2OQ6) in KDM4A (green ribbon and sticks, from PDB ID: 4URA). The catalytic iron, substituted by nickel in the crystal structure (brown sphere) is coordinated by the side chains of E190, H188 and H276. NOG, a close analogue of 2OG, forms one H-bond and one salt bridge to Y132 and K206 respectively (dark blue dashed lines) and bidentate coordination with the metal (light blue dashed lines). Triazole 14a forms similar interactions with the catalytic metal (orange dashed lines), K206 and Y132 (red dashed lines) and is further stabilised by apparent aromatic stacking between the pyridine ring and F185 and van der Waals interactions with S288 (green stick and CPK).* Only the beta strand core of the protein is shown for clarity.