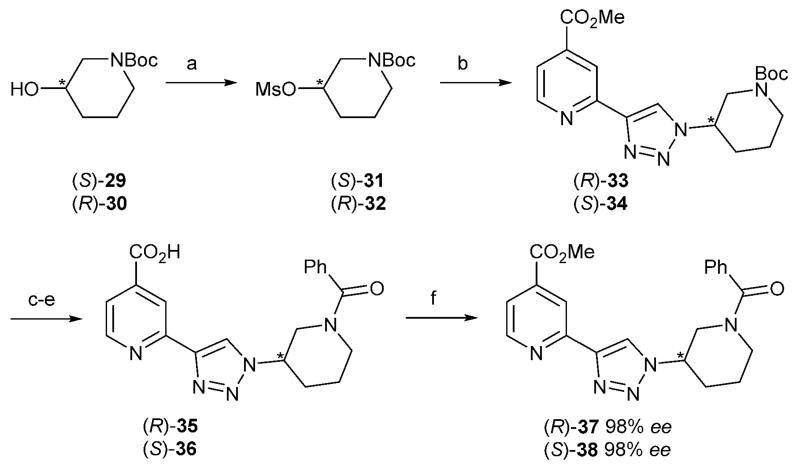
Scheme 5.
Synthesis of the (R)- and (S)-enantiomers of compound 28n and conversion to the methyl ester derivatives. Reagents and conditions: for the (R)-enantiomer: (a) tert-butyl (3S)-3-hydroxypiperidine-1-carboxylate, MsCl, Et3N EtOAc, 96%; (b) (i) NaN3, DMF, 75 °C; (ii) 12, TBAF, DIPEA, CuI, DMF, 48%; (c) TFA, DCM, 97%; (d) PhCOCl, Et3N, MeCN, quant.; (e) LiOH (aq), MeCN, 85%; (f) MeI, NaHCO3, DMF quant. For the (S)-enantiomer: (a) tert-butyl (3R)-3-hydroxypiperidine-1-carboxylate, MsCl, Et3N EtOAc, 98%; (b) (i) NaN3, DMF, 75 °C; (ii) 12, TBAF, DIPEA, CuI, DMF, 34%; (c) TFA, DCM; (d) PhCOCl, Et3N, MeCN; (e) LiOH (aq), MeCN, 81% over 3 steps; (f) MeI, NaHCO3, DMF, 65%.