Figure 6.
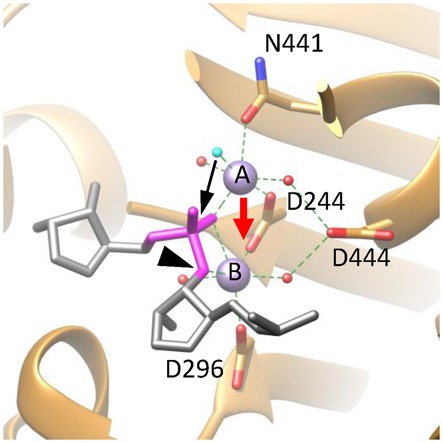
A proposed catalytic mechanism for gp2C. The active site architecture of the DNA-binding state based on the gp2C–K428A:Mn2+:BTP structure (ribbon diagram in gold), in which BTP has been replaced with the DNA substrate fitted as in Figure 5 (stick model in grey) and the nucleophilic water (cyan) that takes the position of the O2 atom of BTP in the gp2C-K428A:Mn2+:BTP structure shown in Figures 4 and 5. For clarity, only the backbone of a strand of the DNA is schematically shown, in which the scissile phosphate is in magenta and the leaving 3′-OH group is indicated with a black arrowhead. Side chains of active side residues are shown as stick models. The two metal ions are shown as purple spheres and are labeled with A and B, respectively. The nucleophilic water is in cyan. Other water molecules, red spheres. Coordination bonds of the metal ions are shown as green dashed lines. The proposed movement of Metal ion A (red arrow) brings the two metal ions closer, generating a positively charged niche that neutralizes, thus helps to stabilize, the highly negatively charged pentacovalent phosphate. The movement of Metal ion A also brings the nucleophilic water (cyan) closer to the scissile phosphate (black arrow) within a distance from the phosphorous that is short enough for formation of a covalent bond. As a result, the nucleophilic attack occurs and the pentacovalent phosphate transition state is formed.
