Figure 1.
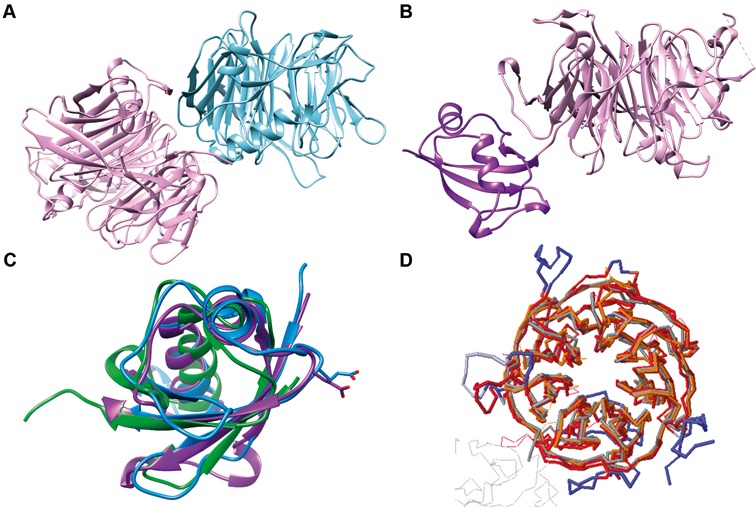
Structural overview of the dimer formed between ChYtm1 and the β-propeller of ChErb1. (A) Global view of the asymmetric unit content. The β-propeller of ChErb1 is shown in blue and ChYtm1 protein is depicted in pink. (B) ChYtm1 protein folds into two separate domains. The amino-terminal region contains 97 residues that form an ubiquitin-like (UBL) domain (in purple), which is attached to the base of a large seven-bladed β-propeller domain (pink). (C) Superimposition of the structures of UBL of ChYtm1 (purple) with ubiquitin (PDB:UBQ1, green) and UBL of ChRsa4 (PDB:4WJS, blue) shows that the ubiquitin-like fold is well preserved. Side chains of the glutamic acid involved in binding to the MIDAS domain of Rea1 are shown for ChYtm1 and ChRsa4. (D) The three most similar structures from PDB (as calculated by Dali Server) were superimposed to the one of ChYtm1100–487. (Chains were colored as follows: 4d6v:A-dark red, 2ymu:A-gray, 4esg:A-orange, ChYtm1-red). Additional segments of ChYtm1 that do not appear in canonical β-propeller fold are represented in blue.
