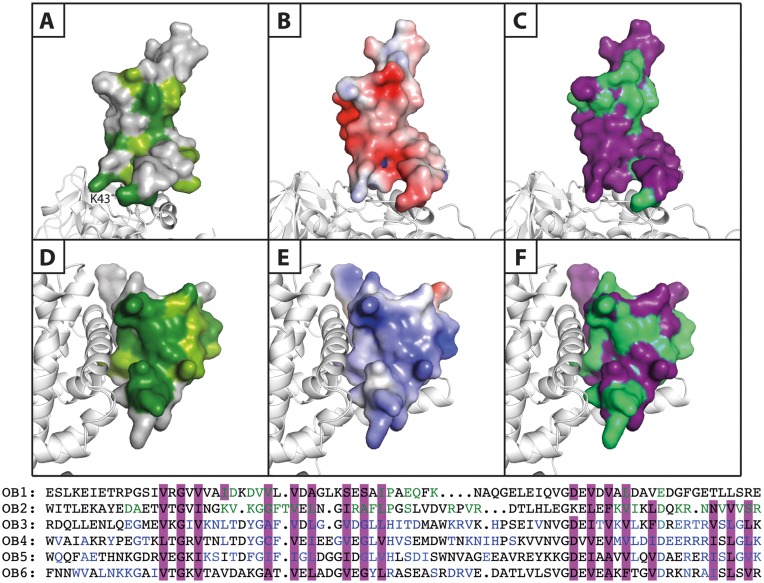
Figure 6.
Comparison of the properties of the OB1 and OB2 β-barrels. Surface representation of the two N-terminal OB domains of S1 bound to the β-subunit presented in white cartoon. Panels A–C illustrate OB1, while panels D–F show OB2. (A and D) Conservation scores of OB1 and OB2 generated using the ConSurf server. Dark green: strictly conserved, lighter green: highly conserved and grey: not conserved. (B and E) Electrostatic surface potentials calculated using the APBS Pymol plugin and mapped onto the separate OB domains. These are shown from negative kbT/ec = –4 (red) to positive kbT/ec = 4 (blue), where kb, T and ec are the Boltzmann's constant, absolute temperature and the charge of an electron, respectively. (C and F) A sequence alignment of the six OB domains of ribosomal protein S1 (OB1–6) was prepared (lower part of the figure) and the residues involved in formation of the β-barrel are highlighted in purple, while the residues with an involvement in general RNA-binding in OB3–6 are coloured blue. Based on this information, the putative RNA-binding residues in OB1 and OB2 are coloured green and mapped onto the OB domains (purple).