Figure 2.
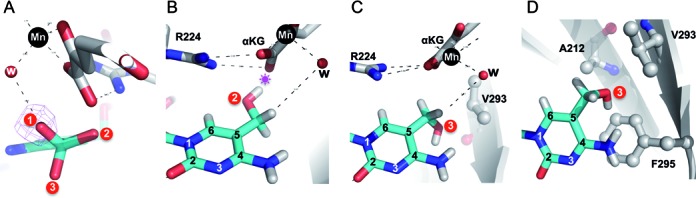
Alternative conformation of 5hmC as a reaction product or substrate. (A) The hydroxyl oxygen atom of 5hmC could adopt three alternative conformations by rotating the C5–CH2 bond every 120°. Conformation 1 represents the experimentally observed product state of 5mC hydroxylation. The metal–ligand water molecule suggests the position where the dioxygen molecule would occupy during the reaction. (B) Conformation 2 would place the hydroxyl oxygen atom of 5hmC in the vicinity of the carboxylate group of α-ketoglutarate (αKG), potentially resulting in repulsion (indicated by a star). (C and D) Two views of conformation 3 with the hydroxyl oxygen atom of 5hmC in close contact with the hydrophobic side chains of A212, V293 and F295.
