Abstract
Objective:
In the present investigation, ethanolic extract of Cyperus rotundus (EECR) rhizomes was evaluated for antidiabetic activity in streptozotocin (STZ)-induced diabetic swiss mice.
Materials and Methods:
After administration of EECR extract for 3 weeks, the body weight, blood glucose, biomarker enzymes (serum glutamic pyruvic transaminase [SGPT] and serum glutamic oxaloacetic transaminase [SGOT]), and plasma lipid levels were measured in STZ-induced diabetic mice.
Results:
The ethanolic extract at dose levels of 250 and 500 mg/kg body weight revealed significant antidiabetic activity, improvement in body weight, and reduction in elevated biochemical parameters such as SGPT, SGOT, cholesterol, and triglyceride levels.
Conclusion:
These experimental findings seemed to indicate the use of this plant in traditional Indian medicine for the treatment of diabetes.
KEY WORDS: Antidiabetic activity, Cyperus rotundus, phytochemical analysis, rhizome, streptozotocin
Diabetes mellitus is a group of syndromes characterized by hyperglycemia; altered metabolism of lipids, carbohydrate and proteins; and an increased risk of complications from vascular diseases. Most patients can be classified clinically as having either Type-1 (insulin dependent diabetes) or Type-2 diabetes mellitus (noninsulin dependent diabetes). In the United States, about 90% of diabetic patients have Type-2 diabetes mellitus. There are more than 125 million persons with diabetes in the world today.[1]
The treatment of diabetes mellitus-2 with oral hypoglycemic agents like sulfonyl urea and biguanides is always associated with numerous side effects.[2] The major advantages of herbal medicine seem to be their good potential, low incidence of serious side effects and low cost.
Cyperus rotundus Linn. (Family - Cyperaceae), commonly known as mustaka, is a perennial weed with dark green glabrous culms, arising from a system of underground tubers.[3,4] The tubers of the plant are used as anthelmintic, antihistaminic, antipyretic, hypotensive, and smooth muscle relaxant.[4] The plant has also been reported to have antimalarial, anti-inflammatory activity, tranquilizing action, as well as hepatoprotective action, against CCl4-induced liver damage.[5]
Materials and Methods
Collection and authentication of plant material
The rhizomes of C. rotundus rhizomes were collected from local area of Moradabad and authenticated by Dr. D.C. Saini, Senior Scientist, Palaeobotany, Birbal Sahni Institute of Palaeobotany, Lucknow, India (voucher specimen no. BSIP03).
Preparation of extract
About 500 g of coarse powder was filled in Soxhlet apparatus for extraction. The whole Soxhlet assembly was set up and first defatted with petroleum ether (60–80°C) and then extracted with ethanol. The petroleum ether and ethanolic extracts were evaporated by using rotatory evaporator and dried on a water bath.
Animals
Swiss albino mice of either sex weighing 25 ± 5 g were housed in clean polypropylene cages and fed with standard pellet diet (Hindustan Lever, Kolkata, India) and water ad libitum. The animal study was performed in Teerthanker Mahaveer College of Pharmacy, TMU, Moradabad, India, after approval from the Institutional Animal Ethics Committee of Teerthanker Mahaveer College of Pharmacy, TMU, Moradabad (Reg. No. 1205/c/08/CPCSEA).
Acute toxicity test
An acute oral toxicity study was performed as per OECD-423 guidelines[6] (acute toxic class method). Albino mice (n = 6) of either sex selected by random sampling technique were kept fasting for overnight with free access to water. The ethanolic extract was administered orally at dose level of 5.0 mg/kg body weight and observed for 14 days, if mortality was observed in 2–3 animals, then the dose administered was assigned as toxic. If mortality was observed in one animal, then the same dose was repeated again to confirm the toxic dose. If mortality was not observed, the procedure was repeated for further higher dose such as 50, 300 and 2000 mg/kg body weight.
Oral glucose tolerance test
Mice divided into three groups (n = 6) were administered with distilled water (10 mL/kg), glibenclamide (10 mg/kg), ethanolic extract of C. rotundus (EECR) at doses of 250 and 500 mg/kg body weight p.o. by oral gavages, respectively. All the animals were given glucose (2.0 g/kg) 60 min after dosing. Blood samples were collected from tail vein just prior to dosing (0 h) and at 30, 60, 90, and 120 min after glucose loading and then glucose levels were estimated.[7,8]
Streptozotocin-induced diabetes
Diabetes was induced in mice by intraperitoneal injection of streptozotocin (STZ) at a dose of 60 mg/kg body weight[7] dissolved in 0.1M cold citrate buffer (pH = 4.5). Diabetes was confirmed by the determination of fasting blood glucose concentration. Mice were divided into five groups of six mice each. Groups I and II served as the vehicle and standard (treated with 10 mg/kg/day glibenclamide). Group III served as diabetic untreated control (STZ). Groups IV and V were treated with extracts at the dose level of 250 mg/kg and 500 mg/kg p.o., respectively, for 21 days. Finally, on day 21, blood was collected to perform various hematological and biochemical parameters. The freshly prepared solutions were orally administered for a period of 21 days. Body weights and blood glucose analysis (with the help of Gluco Check) were done weekly on overnight fasted animals. At the end of the experimental period, the animals were fasted overnight, and blood (0.2 mL) was collected for various biochemical estimations. The animals were sacrificed by cervical decapitation. The liver was dissected, immediately rinsed with ice-cold saline and immediately frozen and stored at −80°C for further biochemical analysis.[8]
Biochemical analysis
Blood samples were centrifuged for 10 min at 7000 rpm using micro-centrifuge to separate the serum and serum glutamic oxaloacetic transaminase (SGOT), serum glutamic pyruvic transaminase (SGPT), serum cholesterol, and triglycerides levels were assessed using standard diagnostic kits.
Statistical analysis
The results were expressed as mean ± standard error mean. The statistical significance was assessed using one-way analysis of variance followed by Bonferroni's multiple comparison test.#P < 0.05 denote value significantly different from control, **P < 0.01, ***P < 0.001 denote value significantly different from diabetic control.
Results and Discussion
The results of the acute oral administration of EECR revealed no mortality up to a dose of 2000 mg/kg body weight (acute toxicity cannot predict long-term administration effect).
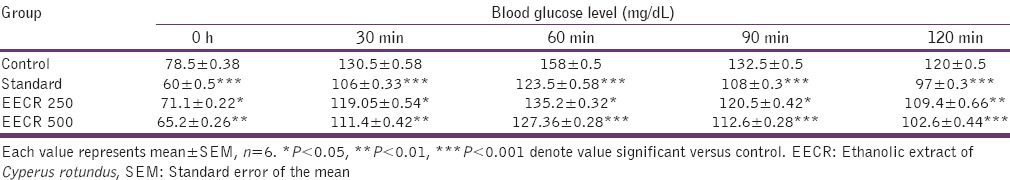
The ethanolic extract (250 and 500 mg/kg body weight) and standard glibenclamide (10 mg/kg body weight) produced significant reduction in elevated blood glucose level at 0 min, 30 min, 60 min, and 120 min when compared to normal control group after glucose administration [Table 1].
Table 1.
Effect of EECR bark on oral glucose tolerance in normal mice
The effect of ethanolic extract and glibenclamide on blood glucose level are shown in Table 2. The ethanolic extract (250 and 500 mg/kg body weight) showed a significant reduction in blood glucose levels in STZ diabetic mice, comparable to that of standard drug glibenclamide at the 21st day of the study. STZ produced significant (P value) loss of body weight as compared to normal animals during the study. The results are shown in Table 3 revealed that diabetic control continued to lose weight till the end of the study, while ethanolic extract (250 and 500 mg/kg body weight) and glibenclamide treated group showed improvement in body weight toward normal value.
Table 2.
Effect of EECR rhizome on fasting blood glucose level in STZ-induced diabetic mice
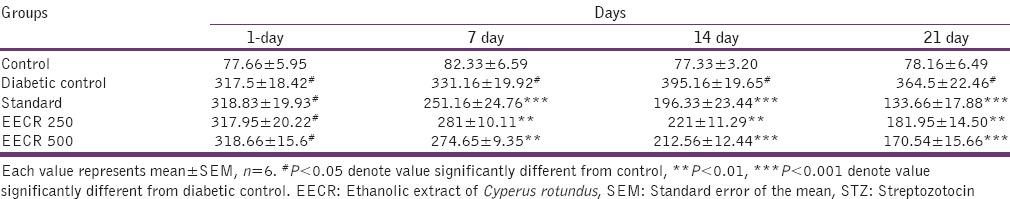
Table 3.
Effect of C. rotunduson body weight of STZ-induced diabetic mice

According to the results given in Table 4, the elevated level of SGPT, SGOT, serum cholesterol, and triglycerides were reduced by ethanolic extract at both doses but the effect was lesser significant than the standard drug. Thus, the extract exhibited antidiabetic activity in STZ-induced diabetic mice, as evident from blood glucose levels.
Table 4.
Effect of EECR rhizome on biochemical parameters in STZ-induced diabetic mice
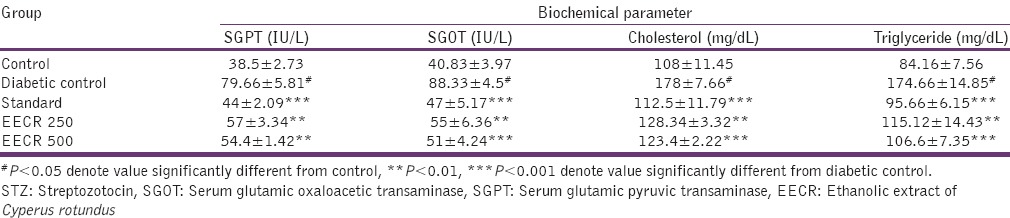
This study was undertaken to evaluate the hypoglycemic activity of EECR in STZ-induced diabetic mice. The animals treated with STZ develop hepatic damage was evident from the increase in enzyme activities. The biomarker enzymes SGOT and SGPT increases due to metabolic changes in the liver (administration of toxins, cirrhosis of the liver, hepatitis diabetes), which results in leakage of enzymes from tissues to the circulation.[9] The administration of EECR and glibenclamide resulted in a decrease in enzyme activities in STZ treated animals. The result indicated that EECR can able to reduce the level of marker enzymes and confirmed the possibility that the ethanolic extract refurbish the function of the liver.
During diabetic state, insulin deficiency inactivates the lipoprotein lipase, which promotes liver conversion of free fatty acids into cholesterol and triglyceride and finally discharged into blood causing elevation of serum cholesterol and triglyceride levels.[7] The administration of EECR significantly reduced serum triglycerides and total cholesterol in STZ-diabetic rats. Thus, it is reasonable to conclude that EECR could modulate blood lipid abnormalities.
Conclusion
The experimental findings of the present study concluded that EECR is capable of exhibiting significant anti-hyperglycemic activity in STZ-induced diabetic mice. The extracts also showed improvement in body weight; biochemical parameters such as SGOT, SGPT, and lipid profile and so might be valuable in diabetes treatment.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Chabner B, Goodman KB, Gilman’s . 10th ed. New York: McGraw-Hill Medical Publication Division; 2001. The Pharmacological Basis of Therapeutics; pp. 1686–9. [Google Scholar]
- 2.Weidmann P, Boehlen LM, de Courten M. Pathogenesis and treatment of hypertension associated with diabetes mellitus. Am Heart J. 1993;125(5 Pt 2):1498–513. doi: 10.1016/0002-8703(93)90447-h. [DOI] [PubMed] [Google Scholar]
- 3.Anonymous . III. New Delhi: CSIR; 1992. The Wealth of India: A Dictionary of Indian Raw Materials and Industrial Products; p. 424. [Google Scholar]
- 4.Nadkarni KM, Nandkarni AK. 3rd ed. I. Mumbai: Popular Prakashan Private Ltd; 1996. Indian Materia Medica; pp. 428–9. [Google Scholar]
- 5.Puratchikody A, Devi CN, Nagalakshmi A. Wound healing activity of Cyperus rotundus Linn. Indian J Pharm Sci. 2006;68:97–101. [Google Scholar]
- 6.Ecobichon DJ. New York: RC Press; 1997. The Basis of Toxicology Testing; pp. 43–86. [Google Scholar]
- 7.Gupta RK, Kumar D, Chaudhary AK, Maithani M, Singh R. Antidiabetic activity of Passiflora incarnata Linn. in streptozotocin-induced diabetes in mice. J Ethnopharmacol. 2012;139:801–6. doi: 10.1016/j.jep.2011.12.021. [DOI] [PubMed] [Google Scholar]
- 8.Chakravarty S, Kalita JC. Antihyperglycaemic effect of flower of Phlogacanthus thyrsiflorus Nees on streptozotocin induced diabetic mice. Asian Pac J Trop Biomed. 2012;(Suppl 3):S1357–61. [Google Scholar]
- 9.Chalasani N, Aljadhey H, Kesterson J, Murray MD, Hall SD. Patients with elevated liver enzymes are not at higher risk for statin hepatotoxicity. Gastroenterology. 2004;126:1287–92. doi: 10.1053/j.gastro.2004.02.015. [DOI] [PubMed] [Google Scholar]


