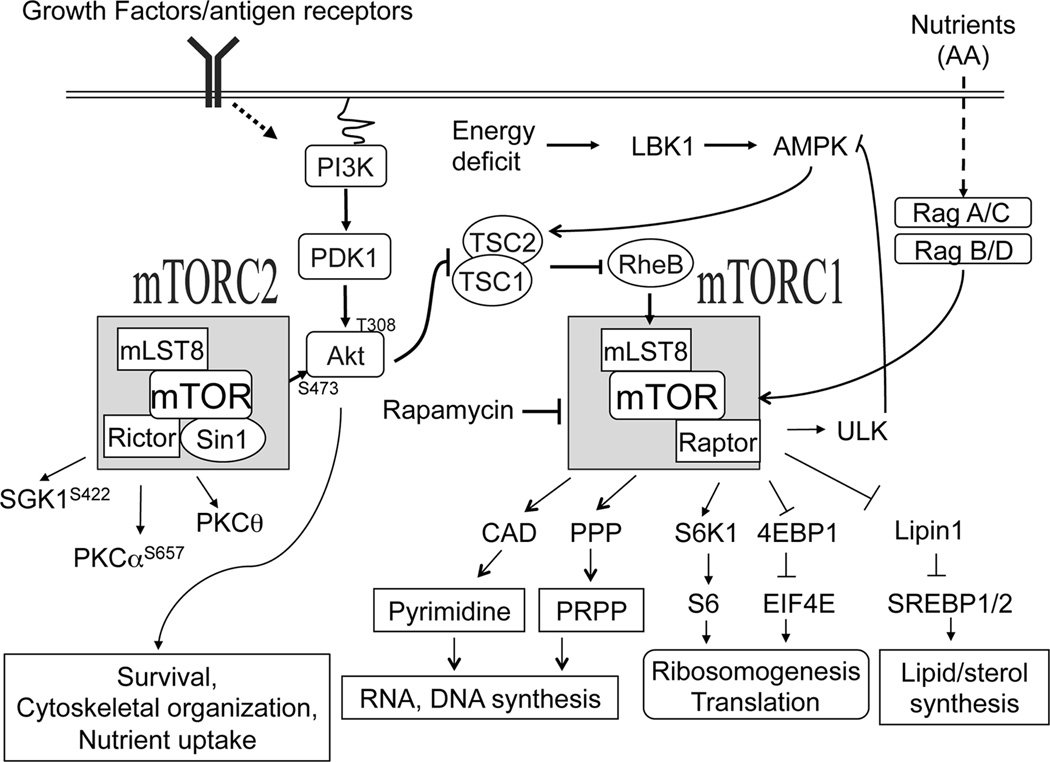
Figure 3. TSC1/2-mTOR signaling.
mTOR signals through two functionally distinct complexes: mTORC1 and mTORC2. In addition to mTOR and several other components, mTORC1 and mTORC2 contain distinct adaptor molecules raptor and rictor, respectively. mTOR senses and integrates various extracellular signals such as growth factor, cytokines, immune receptor, amino acids and intracellular signals including stress and energy levels. The Small GTPase Rheb in its GTP bound active state directly activates mTORC1. TSC1/2 complex acts as a negative regulator of mTORC1 via the GAP activity of TSC2 to RheB. Growth factors and antigen receptors signal through the PI3K-PDK-Akt pathway to activate mTORC1 and mTORC2. In contrast, nutrients activate mTORC1 through Rag GTPases. Cellular energy deficits can take a direct route to suppress mTORC1 activation through LKB1 and AMPK, which promote TSC2 activity. mTORC1 phosphorylate multiple targeting proteins such as S6K1, 4E-BP’s, Lipin1, CAD (carbamoylphosphate synthetase, aspartate transcarbamoylase, and dihydroorotase), and 5’-hosphoribosyl-1’-pyrophosphate (PRPP) that promote anabolic processes such as protein synthesis, lipid/sterol synthesis and de novo pyrimidine synthesis. mTORC1 also prevents autophagy indirectly by inhibiting AMPK through direct phosphorylation of ULK1. Although PI3K is important for mTORC2 activation, the exact mechanisms that activate mTORC2 remain elusive. mTORC2 also phosphorylates multiple intracellular signaling molecules such as Akt at the serine 473 residue, PKCα at S657, PKCθ at S660/676, and SGK at S422 to regulate cell survival, nutrient uptake, cytoskeletal organization, and Th differentiation.