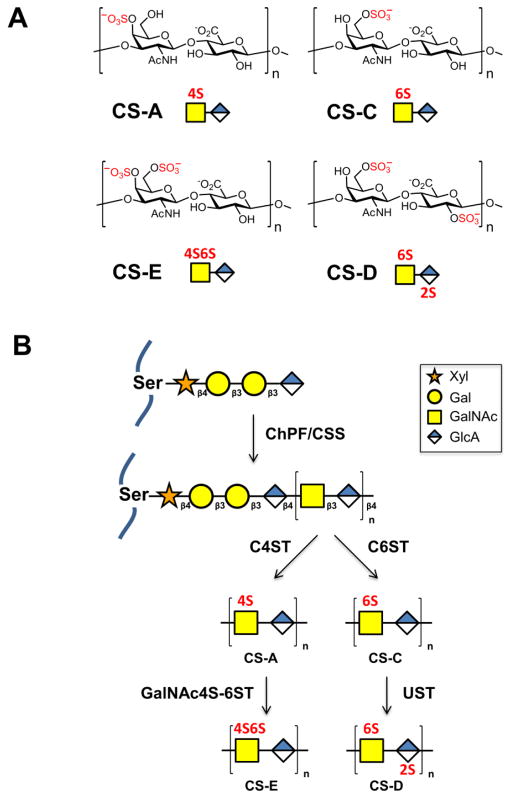
Figure 1.
(A) Common sulfation motifs of chondroitin sulfate, which consists of the repeating disaccharide N-acetyl-D-galactosamine-β(1,3)-D-glucuronic acid. n = 20–100. CS-A and CS-C are monosulfated at the 4-O and 6-O positions of GalNAc, respectively. CS-D is sulfated at the 2-O position of GlcA and 6-O position of GalNAc. CS-E is sulfated at the 4-O and 6-O positions of GalNAc. (B) Biosynthesis of chondroitin sulfate. A core tetrasaccharide (xylose (Xyl)-galactose (Gal)-galactose (Gal)-glucuronic acid (GlcA)) is appended to serine residues of the core proteoglycan. Chain extension is performed by chondroitin sulfate synthase (CSS) and chondroitin polymerizing factor (ChPF). The polysaccharide chains are then elaborated through sulfation by C4ST to generate CS-A or C6ST to generate CS-C, followed by GalNAc4S-6ST or UST to form CS-E or CS-D, respectively.