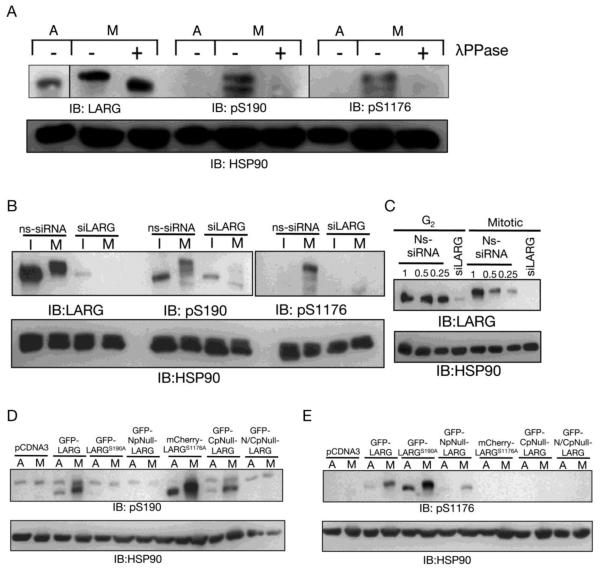
Figure 4. Phosphospecific antibodies reveal that LARG is phosphorylated at S190 and S1176 during mitosis.
(A) Phosphoantibody specificity for phosphorylated LARG was determined by phosphatase treatment of mitotic cell lysates. Cell lysates were prepared from asynchronous HeLa cells (A) and nocodazole-arrested mitotic HeLa cells (M). As described in Materials and Methods, mitotic cell lysates were treated with λ protein phosphatase (+). Then lysates were immunoblotted with anti-LARG, anti-pS190 LARG, and anti-pS1176 antibodies, as indicated. (B) Phosphoantibody specificity for LARG was determined by siRNA knockdown of LARG. HeLa cells were treated with non-specific (ns) siRNA and siRNA specific for LARG (siLARG), as described previously [9]. After nocodazole treatment to arrest cells in prometaphase, mitotic cells were harvested by shake-off and lysates were prepared (M). Lysates were also prepared from the G2/interphase cells remaining on the culture dish (I). Lysates were then immunoblotted with anti-LARG, anti-pS190 LARG, and anti-pS1176 LARG antibodies, as indicated. (C) Immunoblot showing efficiency of LARG siRNA. Lysates were prepared from G2/interphase (G2) and mitotic cells (M) treated with Ns-siRNA or LARG siRNA, as described above and used in (B), followed by immunoblotting with an anti-LARG antibody. Ns lysates were used at 1, 0.5 and 0.25 equivalents compared to siLARG lysates to show efficient knockdown (≥90%) of endogenous LARG. (D and E) Phosphoantibodies were tested on LARG point and multi-site phosphomutants. HeLa cells were transfected with the indicated constructs, treated with nocodazole, and subjected to mitotic shake-off. Cell lysates from the mitotic cells (M) and asynchronous cells (A) were prepared and immunoblotted using anti-pS190 LARG, anti-pS1176 LARG and anti-HSP90 (loading control) antibodies, as indicated.