Fig. 10.
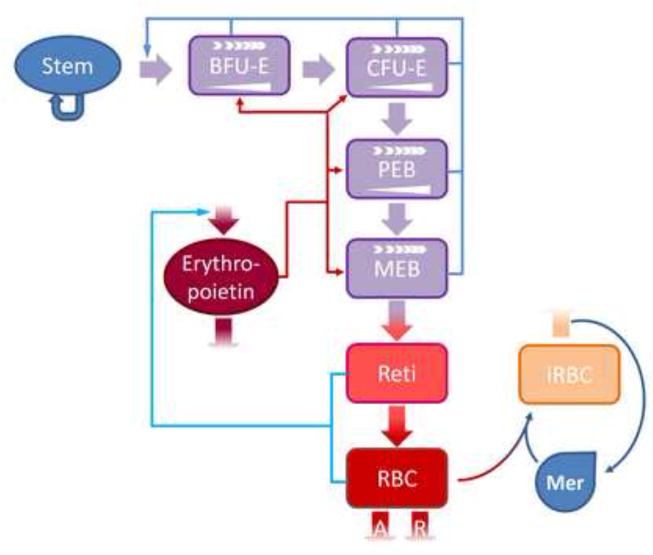
Scheme of a DRE model with age classes for erythropoiesis and blood-stage malaria infection that corresponds to Schirm’s erythropoietic model [27]. The malaria blood-stage infection is modeled with a Lotka-Volterra model. In this model, stem cells (Stem) constitute a self-sustained pool that also generates burst forming unit-erythroids (BFU-E). These differentiate sequentially into colony forming unit-erythroids (CFU-E), proliferating erythrocytic blasts (PEB) and maturing erythrocytic blasts (MEP). MEP give rise to circulating reticulocytes (Reti), which finally differentiate into circulating RBCs. Erythropoiesis is modeled with three regulatory loops. RBCs and reticulocytes transport oxygen, which negatively regulates the production of erythropoietin (shown in green). Erythropoietin also regulates the amplification (
 ) of BFU-E, CFU-E and PEB and the rate of differentiation (
) of BFU-E, CFU-E and PEB and the rate of differentiation (
 ) of BFU-E, CFU-E, PEB and MEB (shown in scarlet thin arrows); in turn, the number of progenitor and precursor cells in the bone marrow negatively regulates the number of stem cells (shown in turquoise). RBCs are subject to random, age-independent loss (R), in addition to the natural age-dependent loss (A) at the end of their lifespan. When present, merozoites (Mer) can infect RBCs of certain age classes. At the end of their lifespan, the infected RBCs (iRBC) burst, giving rise to 8–32 new merozoites per iRBC.
) of BFU-E, CFU-E, PEB and MEB (shown in scarlet thin arrows); in turn, the number of progenitor and precursor cells in the bone marrow negatively regulates the number of stem cells (shown in turquoise). RBCs are subject to random, age-independent loss (R), in addition to the natural age-dependent loss (A) at the end of their lifespan. When present, merozoites (Mer) can infect RBCs of certain age classes. At the end of their lifespan, the infected RBCs (iRBC) burst, giving rise to 8–32 new merozoites per iRBC.
