Abstract
Persistent myofibroblast differentiation is a hallmark of fibrotic diseases. Myofibroblasts are characterized by de novo expression of alpha smooth muscle actin (αSMA) and excess fibronectin assembly. Recent studies provide conflicting reports on the effects of tyrosine kinase inhibitor dasatinib on myofibroblast differentiation and fibrosis. Also, it is not fully understood whether dasatinib modulates myofibroblast differentiation by targeting Src kinase. Herein, we investigated the effect of dasatinib on cSrc and transforming growth factor-β (TGFβ)-induced myofibroblast differentiation in vitro. Our results indicated that selective Src kinase inhibition using PP2 mimicked the effect of dasatinib in attenuating myofibroblast differentiation as evident by blunted αSMA expression and modest, but significant inhibition of fibronectin assembly in both NIH 3T3 and fibrotic human lung fibroblasts. Mechanistically, our data showed that dasatinib modulates αSMA synthesis through Src kinase-mediated modulation of serum response factor expression. Collectively, our results demonstrate that dasatinib modulates myofibroblast differentiation through Src-SRF pathway. Thus, dasatinib could potentially be a therapeutic option in fibrotic diseases.
Keywords: Dasatinib, Src, TGFβ, Myofibroblast, Serum response factor
1. Introduction
Over the past three decades, myofibroblasts have emerged as the central effector cells in wound healing and tissue fibrosis. Myofibroblasts promote abnormal hypertrophic scar formation process, which is characteristic of tissue fibrosis (Hinz et al., 2010). A hallmark of fibroblast activation into myofibroblasts is the de novo expression of alpha smooth muscle actin (αSMA) and persistent extracellular matrix (ECM) accumulation (Hinz et al., 2012; Tomasek et al., 2002). Finding effective therapeutics remains a challenge due to the evolving paradigm in the pathogenesis of fibrotic diseases. While the mechanisms of the pathologic activation of fibroblasts are not completely understood, transforming growth factor β (TGFβ), pro-fibrotic cytokine, is a well-established trigger and promoter of persistent myofibroblast differentiation (Hinz et al., 2010; Tomasek et al., 2002). Previous studies have also implicated a pro-fibrotic role of Src kinases in the non-canonical signaling of TGFβ and in mediating fibroblast adhesion, migration, and myofibroblast-mediated ECM assembly (Hu et al., 2014; Schlaepfer et al., 1997; Skhirtladze et al., 2008). Src kinases are involved in fibroblast adhesion to the ECM via regulation of adhesion proteins such as FAK (Okutani et al., 2006; Vittal et al., 2005). This discrepancy on the effects of dasatinib and Src kinases on pulmonary fibrosis raises questions if dasatinib indeed mediates its effects on pulmonary fibrosis through activity modulation of Src kinases.
Dasatinib selectively targets Src family of kinases, Bcr-Abl and PDGF receptors, and is currently approved for the treatment of a variety of neoplasias (Kantarjian et al., 2006; Montero et al., 2011; Roskoski, 2015). Dasatinib has shown beneficial effects on reducing ECM production in systemic sclerosis through c-abl and Src modulation (Skhirtladze et al., 2008). However, the role of dasatinib in myofibroblast differentiation is not fully understood. Since TGFβ has been shown to activate Src signaling in fibroblasts and Src is a major regulator of profibrotic adhesion proteins, we hypothesized that targeting Src kinases using dasatinib may ameliorate myofibroblast differentiation and ECM fibronectin accumulation. In this study, the regulatory role of dasatinib on myofibroblast differentiation and ECM accumulation relevant to fibrosis were studied in mouse embryonic fibroblasts (NIH 3T3 cells), human primary lung fibroblasts (HLFs) and human fibrotic lung fibroblasts (HFLFs). We found that dasatanib significantly decreased αSMA expression and mimicked the effects of selective Src family kinases inhibitor, PP2. Additionally, similar to PP2, dasatinib decreased fibronectin matrix assembly by NIH 3T3 and HFLFs. We also found that dasatinib mediated these effects via modulation of Src signaling and the expression of transcription factor serum response factor (SRF). Our results indicate that targeted Src kinase inhibition using dasatinib could potentially be a therapeutic option in patients with organ fibrosis including IPF.
2. Material and Methods
2.1.Cell Lines and Cell Culture
NIH 3T3, HLFs, and HFLFs were obtained from ATCC (Manassas, VA). To examine optimal time for myofibroblast differentiation, NIH 3T3 and HLFs were cultured on 6-well plates, after reaching 70% confluence, they were subjected to serum starvation in the presence or absence of 100 pM recombinant TGFβ (R&D Systems, Minneapolis, MN), a pre-determined dose (Abdalla et al., 2013; Goc et al., 2011) for 48, or 72 h. Cells were subjected to Western analyses as described below. For the mechanistic pharmacologic inhibition studies: after reaching 70% confluence, NIH 3T3 cells were treated with control PBS or TGFβ (100 pM) for 48 h. This was followed by co-treatment for 24 h (total 72 h) with inhibitor of Src family kinases using PP2 (2.5, 5, 10, or 25 µM) obtained from Sigma (St. Louis, MO) or dasatinib (2.5, 5, 10, or 25 nM) obtained from Santacruz Biotechnology (Dallas, TX). Cells were subjected to Western analyses as described below. Our finding from mouse NIH 3T3 were confirmed in HFLFs isolated from an IPF patient. HFLFs were subjected to 2% FBS and treated with PP2 (10 or 40 µM) or dasatinib (10 or 40 nM). Cells were subjected to Western analyses as described below.
2.2.Antibodies
Anti-αSMA (catalog number SAB2500963) and anti-fibronectin (catalog number F6140) antibodies for Western analysis were purchased from Sigma (St. Louis, MO). GAPDH (catalog number 2118), and anti-SRF (catalog number 5147) antibodies were purchased from Cell Signaling (Boston, MA). Anti-phospho-SrcTyr416, pan-cSrc, phospho-GSK-3α/βSer9/21, phospho-βcateninSer33/37/Thr41, were purchased from Cell Signaling (Boston, MA). Anti-fibronectin (catalog number ab2413) and anti-αSMA (catalog number ab5694) antibodies for immunofluorescence imaging were purchased from Abcam (Cambridge, MA).
2.3.Western Blot Analysis
Cell lysates were prepared using lysis buffer (20 mM Tris-HCl, pH 7.4, 1% Triton X-100, 3 mM EGTA, 5 mM EDTA, phosphatase inhibitors (10 mM sodium pyrophosphate, 5 mM sodium orthovanadate, 5 mM sodium fluoride, and 10 µMokadaic acid), protease inhibitor mixture (Roche Diagnostics) and 1 mM PMSF). SDS-PAGE and Western blotting were performed as described previously (Abdalla et al., 2013)
2.4.Immunocytochemistry
Immunofluorescence staining was performed as described previously (Abdalla et al., 2013; Somanath et al., 2007). Briefly, NIH 3T3 and HFLFs were plated on 8-well chamber slides. After reaching 70% confluence, NIH 3T3 cells were subjected to serum starvation in the presence or absence of TGFβ for 48 h and co-treated with PP2 (10 or 40 µM) or dasatinib (10 or 40 nM) for an additional 24 h (72 h total). HFLFs were subjected to 2% FBS serum starvation and treated with PP2 (10 or 40 µM) or dasatinib (10 or 40 nM) for 24 h. Next, cells were fixed with 2% paraformaldehyde in 1× PBS followed by permeabilization with 0.1% Triton X-100 in 1× PBS. The nonspecific staining was blocked with 2% BSA for 1 h at room temperature. The fixed and permeabilized cells were incubated with primary anti-αSMA antibody (Abcam) (dilution 1:1000) or anti-fibronectin antibody (Abcam) (dilution 1:1000) overnight at 4 °C and washed. Secondary Alexa Fluor 488-labeled antibody was applied for 1 h. Secondary antibodies were also tested for any non-specific staining in NIH-3T3 cells and HFLF. The slides were mounted with Vectashield with DAPI (Vector Laboratories, PA), and imaged by a Zeiss fluorescent microscope.
2.5.Statistical Analysis
All data are presented as mean ± S.D. To determine significant differences between treatment and control values, we used the Student's two-tailed t test. The significance was set at 0.05 levels (marked with symbols for data that are statistically significant).
3. Results
3.1.Dasatinib attenuated TGFβ-induced αSMA expression
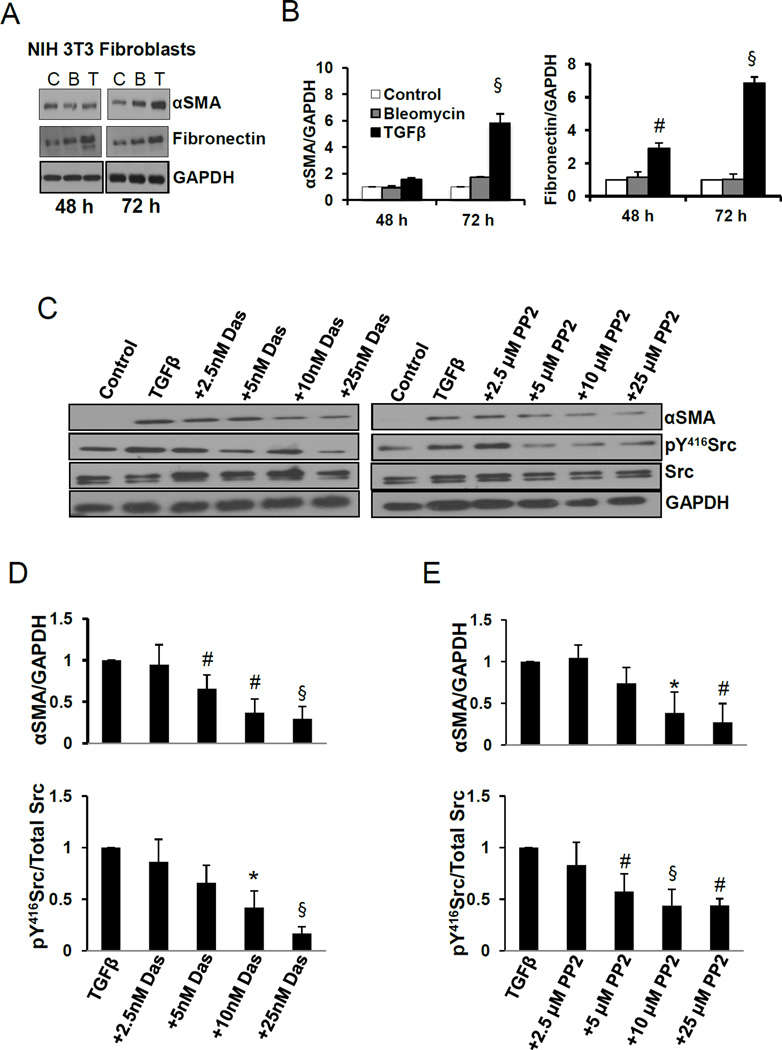
We first determined the optimal time of myofibroblast differentiation in response to TGFβ as measured by αSMA. TGFβ (100 pM) induced a significant increase in αSMA expression in a time-dependent manner that peaked at 72 h in serum starved NIH 3T3 fibroblasts (Fig. 1A and B). Similar effects were also observed in primary human lung fibroblasts (HLFs) (Supplemental Fig. 1A–C). Next, we determined the role of dasatinib on TGFβ-induced αSMA expression and whether it is mediated through Src pathway. Our results indicated that dasatinib significantly decreased TGFβ-induced αSMA expression in a dose dependent manner, which correlated with decreased Src phosphorylation (Fig. 1 C and D). Similarly, treatment with increasing concentrations of PP2 decreased TGFβ-induced αSMA expression and was associated with decreased Src phosphorylation (Fig. 1 D and E).
Fig. 1.
Dasatinib inhibits TGFβ-induced myofibroblast differentiation and αSMA expression. A. Western blot images of NIH 3T3 lysates treated in the presence and absence of 100 pM TGFβ or 2.5 mU bleomycin for 48, and 72 h, probed for αSMA and fibronectin. B. Densitometry analysis of the Western bands showing expression changes in αSMA and fibronectin with 100 pM TGFβ and 2.5 mU bleomycin for 48, and 72 h and normalized to GAPDH (n=3–6). C. Western blot images of NIH 3T3 lysates treated in the presence and absence of 100 pM TGFβ (48 h) and in combination of TGFβ (48 h total) with various doses of dasatinib or Src inhibitor PP2 (24 h), probed for αSMA, pY416Src and total Src. D. Densitometry analysis of the Western bands showing expression changes in αSMA and pY416Src with 100 pM TGFβ combined with various doses of dasatinib, and normalized to GAPDH and total Src, respectively. (n=3). E. Densitometry analysis of the Western bands showing expression changes in αSMA and pY416Src with 100 pM TGFβ combined with various doses of Src inhibitor PP2 and normalized to GAPDH and total Src, respectively (n=3). Data presented as mean ± S.D. *P<0.05; #P<0.01; §P<0.001
3.2.Dasatinib inhibited TGFβ-induced αSMA expression through a Src and transcription factor SRF pathway
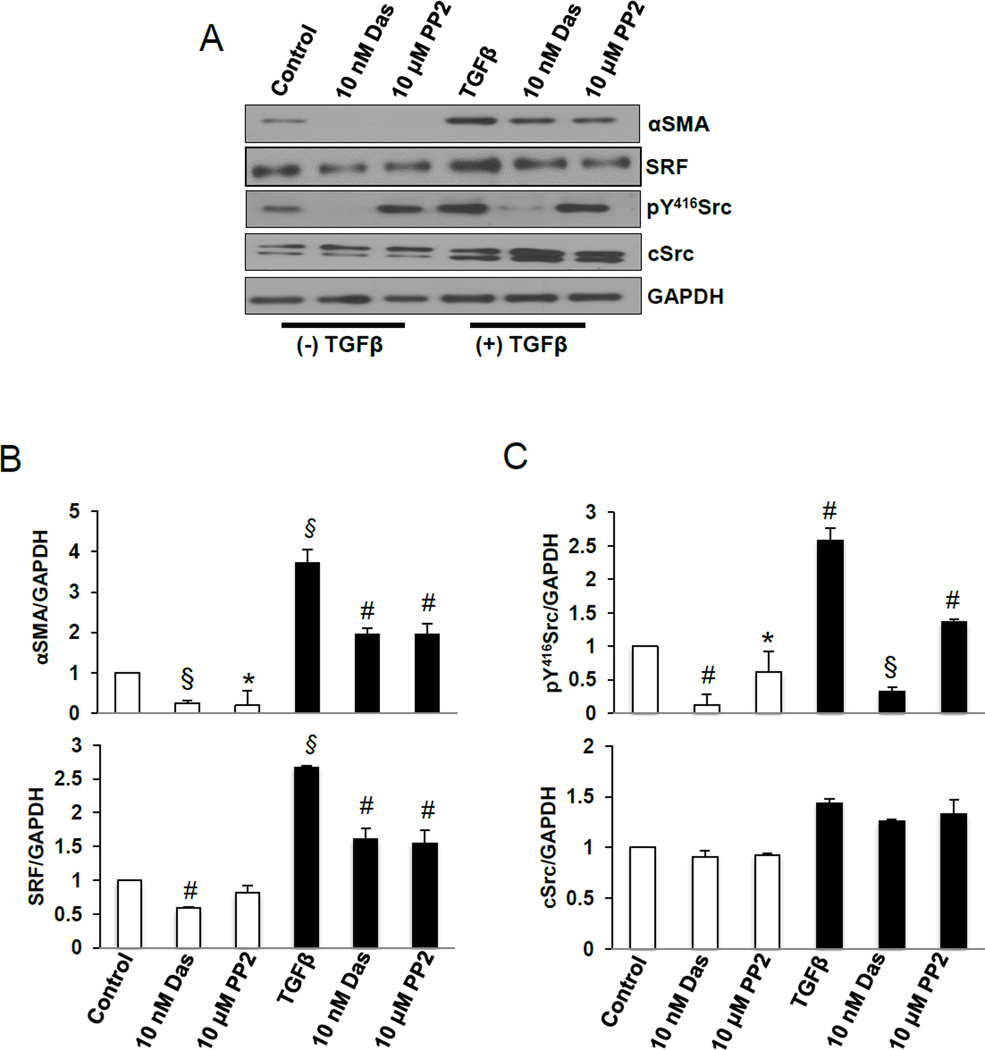
To identify the mechanism by which dasatinib modulates TGFβ-induced αSMA synthesis, we sought to explore whether SRF is a down-stream effector of Src signaling pathway. Our results indicated that, compared to untreated cells, TGFβ-induced αSMA expression correlated with increased SRF expression and Src phosphorylation (Fig. 2A). Treatment with dasatinib or PP2 significantly decreased αSMA expression which was associated with moderate decrease in SRF expression and Src phosphorylation (Fig. 2 A–C).
Fig. 2.
Dasatinib inhibits αSMA synthesis, Src pY416 phosphorylation in NIH 3T3 fibroblasts. A. Western blot images of NIH 3T3 lysates treated in the presence and absence of 100 pM TGFβ (48 h) and TGFβ (48 h total) in combination with 10 nM dasatinib or 10 µM PP2 (24 h), probed αSMA, SRF, pY416Src and total Src. B. Densitometry analysis of the Western bands showing expression changes in αSMA and SRF with 100 pM TGFβ (48 h) alone and in combination with 10 nM dasatinib or 10 µM PP2 (24 h), and normalized to GAPDH. (n=3). C. Densitometry analysis of the Western bands showing expression changes in pY416Src and total Src with 100 pM TGFβ (48 h) alone and in combination with 10 nM dasatinib or 10 µM PP2 (24 h), normalized to GAPDH (n=3). Data presented as mean ± S.D. *P<0.05; #P<0.01; §P<0.001
3.3.Dasatinib inhibited TGFβ-induced fibronectin matrix assembly
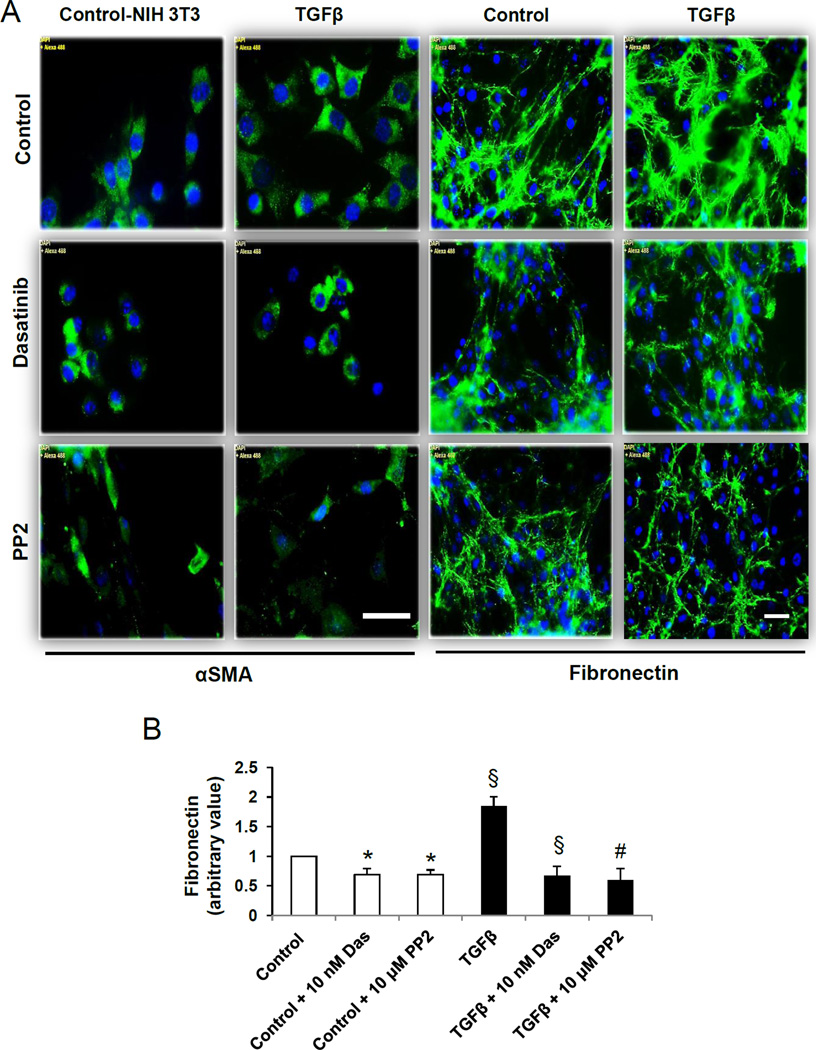
Next, we sought to investigate whether dasatinib modulates αSMA staining and fibronectin matrix assembly in NIH 3T3 fibroblasts in the absence or presence of TGFβ. Compared to unstimulated NIH 3T3 fibroblasts, TGFβ induced marked increase in both αSMA expression and fibronectin matrix assembly (Fig. 3 A and B). This effect was blunted upon treatment with dasatinib or PP2 (Fig. 3 A and B).
Fig. 3.
Dasatinib inhibits TGFβ-induced αSMA expression and fibronectin assembly in NIH3T3 fibroblasts. A. Representative images of NIH3T3 fibroblast layers subjected to serum starvation followed by treatment with 100 pM TGFβ (48 h) and in combination with 10 nM dasatinib or 10 µM PP2 (24 h), followed by probing with anti-αSMA and anti-fibronectin primary antibodies as well as Alxa Flour 488-labeled secondary antibodies. B. Bar graph showing NIH-Image J based quantitative analysis of assembled fibronectin by NIH 3T3 fibroblasts in the presence or absence of TGFβ and co-treatment with 10 nM dasatinib or 10 µM PP2 in serum free medium (n=3). Data presented as mean ± S.D. Scale bar: 50 µm. *P<0.05; #P<0.01; §P<0.001
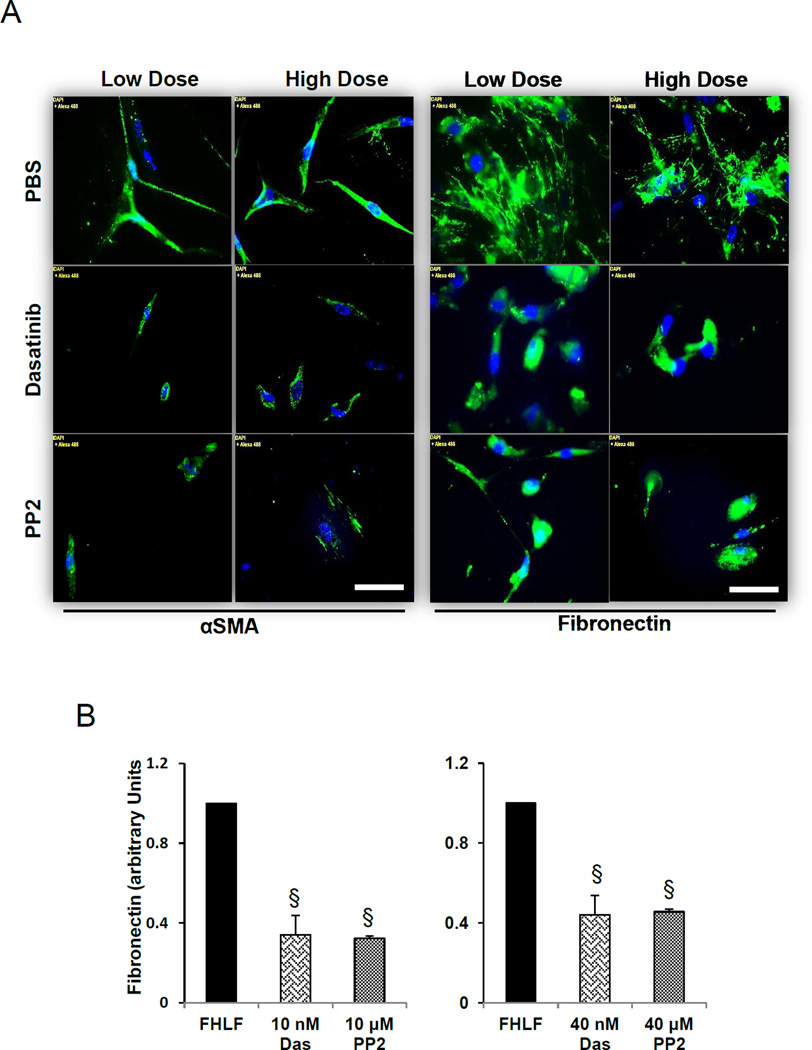
3.4.Dasatinib attenuated αSMA and fibronectin expression and assembly in HFLFs
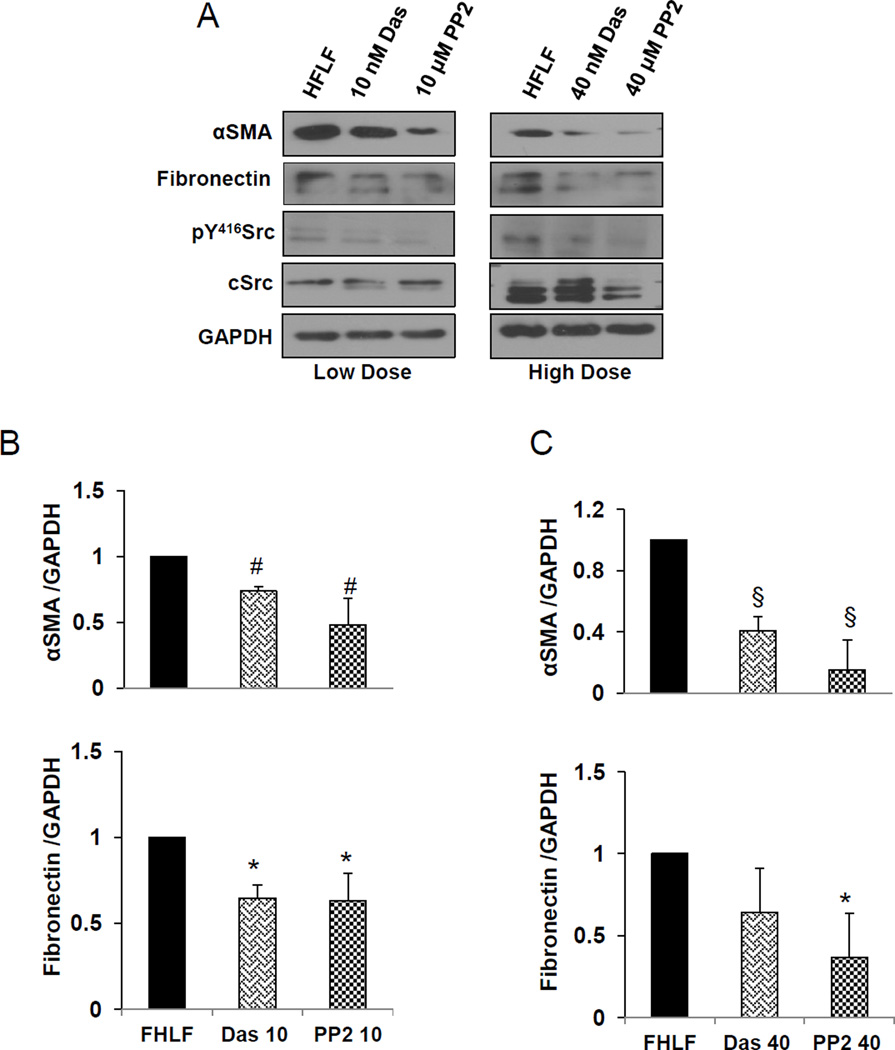
Next we asked whether dasatinib can modulate stable HFLFs isolated from IPF patient lungs and how this effected compared to PP2. To account for HFLFs resilience compared to NIH 3T3, we utilized 2 doses of dasatinib (10 and 40 nM) and PP2 (10 and 40 µM). Treatment with dasatinib or PP2 resulted in a significant decrease in αSMA expression and was associated with decreased fibronectin expression: this effect was augmented in HFLFs treated with high concentration dasatinib or PP2 (Fig. 4 A–C). These effects were further confirmed using immunostaining of HFLF cells in culture (Fig. 5 A and B).
Fig. 4.
Dasatinib inhibits αSMA and fibronectin expression in HFLFs through Src. A. Western blot images of HFLFs maintained in 2% serum (partial serum starvation) and treated with 10 nM dasatinib or 10 µM PP2 (low dose) or 40 nM dasatinib and 40 µM PP2 (high dose) for 24 h, and the lysates probed with antibodies against αSMA, fibrinectin, pY416Src and total cSrc. B. Bar graphs of showing densitometry analysis of HFLF lysate Western blot images after treatment with 10 nM dasatinib or 10 µM PP2 (low dose) or 40 nM dasatinib and 40 µM PP2 (high dose) for 24 h showing expression changes in αSMA, fibronectin, pY416Src and total cSrc. (n=3). Data presented as mean ± S.D. *P<0.05; #P<0.01; §P<0.001
Fig. 5.
Dasatinib inhibits αSMA and fibronectin assembly in HFLFs. A. Representative images of HFLFs subjected to serum starvation (in 2% serum) followed by treatment with 10 nM dasatinib or 10 µM PP2 (low dose) or 40 nM dasatinib or 40 µM PP2 (high dose) for 24 h, and probed for αSMA and fibronectin expression using Alxa Flour 488-labeled secondary antibodies. B. Bar graph showing NIH-Image J based quantitative analysis of SMA expression (left) and fibronectin assembly (right) by HFLFs after low and high dose treatments with dasatinib or PP2 in 2% serum medium (n=3). Data presented as mean ± SD. Scale bar: 50 µm. * §P<0.001
4. Discussion
Our studies revealed novel anti-fibrotic properties of dasatinib as it inhibits myofibroblast differentiation in addition to modulating ECM fibronectin expression and assembly with potential therapeutic implications in fibrotic diseases such as pulmonary fibrosis. First, dasatinib decreased TGFβ-induced αSMA expression in a dose dependent manner and mimicked the effects of the selective Src family kinase inhibitor, PP2. Second, similar to PP2, dasatinib ameliorates αSMA expression and fibronectin assembly in TGFβ-stimulated mouse NIH 3T3 fibroblasts and HFLFs isolated from a patient diagnosed with idiopathic pulmonary fibrosis. Collectively, our results demonstrate that dasatinib ameliorates persistent myofibroblast differentiation and ECM accumulation, two hallmark events in tissue fibrosis. Thus, this suggests that dasatinib has favorable anti-fibrotic effects and may serve as a potential therapeutic strategy in patients with organ fibrosis including pulmonary fibrosis.
Myofibroblasts, marked by de novo expression of αSMA, are principle orchestrators in the fibrogenesis of organs such as lung and liver (Gressner et al., 2006; Hinz et al., 2010; Tomasek et al., 2002). Persistent extracellular matrix accumulation is a hallmark of myofibroblast differentiation resulting in tissue fibrosis (Tomasek et al., 2002). Previous studies have implicated a role for Src kinases in the non-canonical signaling induced by TGFβ, a potent pro-fibrotic cytokine, and in mediating cell adhesion to the ECM (Hu et al., 2014; Schlaepfer et al., 1997; Skhirtladze et al., 2008). Src kinases modulate cell adhesion, proliferation, and migration by orchestrating the dynamic interaction between resident fibroblasts and the ECM via regulation of integrins and focal adhesion proteins (Pechkovsky et al., 2008; Schlaepfer et al., 1997; Thannickal et al., 2003). Additionally, a study has demonstrated that TGFβ-mediated Collagen type I synthesis, secretion and assembly in response to myofibroblast transdifferentiation is dependent on Src activation (Mishra et al., 2007). Interestingly, TGFβ-medicated regulation of Src has been shown to be cell type- and environment-specific resulting in increased (Kim et al., 2005; Mishra et al., 2007; Park et al., 2004; Pechkovsky et al., 2008) or decreased (Atfi et al., 1994; Fukuda et al., 1998; Manganini et al., 2000) Src activity.
The pro-fibrotic effects of Src kinases are of particular clinical interest as they could be novel targets in the management of organ fibrosis using tyrosine kinase inhibitors (TKIs) such as imatinib, dasatinib, and nintedanib. Imatinib, a first-generation TKI selective to PDGFR and c-Abl, was shown to ameliorate myofibroblast differentiation and pulmonary fibrosis in several animal models (Aono et al., 2005; Daniels et al., 2004; Li et al., 2009; Rice et al., 1999). However, in phase II clinical trial, imatinib failed to yield significant improvements in lung function or survival rate (Daniels et al., 2010). On the other hand, nintedanib, a multi-targeted TKI (targets PDGFR, FGFR, VEGFR, TGFβ, c-Abl and Src family kinases) exhibited significant therapeutic effects on modulating myofibroblast differentiation and ECM secretion and assembly in vitro (Hostettler et al., 2014; Wollin et al., 2014), attenuating pulmonary fibrosis in vivo (Wollin et al., 2014), and improving pulmonary function and quality of life in clinical trials (Richeldi et al., 2014); nintedanib was FDA approved for IPF management in 2014. The clinical outcomes observed with imatinib and nintedanib underscores the exclusion of class effect across all TKIs and that mechanism of action of each TKI differs within the class.
Noteworthy, Vittal and colleagues have shown that imatinib failed to modulate TGFβ-induced αSMA expression and did not protect against bleomycin-induced pulmonary fibrosis (Vittal et al., 2007). Compared to imatinib, our results demonstrate that dasatinib is a potent inhibitor of TGFβ-induced αSMA synthesis, a marker for myofibroblast differentiation, and ECM fibronectin assembly by targeting Src signaling. Our results are supported by similar findings that were reported with saracatinib (Hu et al., 2014). Another important difference is the effect on Src signaling; studies have shown that imatinib has a much lower selectivity and binding affinity for cSrc over c-Abl due to the lack of flipped conformation of DFG (Asp-Phe-Gly) motif on Src’s kinase domain (Lin et al., 2013; Seeliger et al., 2007; Seeliger et al., 2009). Our studies demonstrate that dasatinib mediates its effects on myofibroblasts differentiation and the ECM by targeting Src signaling; this mimicked the effects of PP2, a selective Src kinase inhibitor. This supports previous findings by Mishra and colleagues that pharmacological inhibition and genetic downregulation of Src prevented Collagen type I synthesis, secretion and assembly (Mishra et al., 2007). Interestingly, we found that targeting Src using dasatinib was associated with decreased SRF which suggests that Src may transcriptionally regulates αSMA synthesis. In light of this finding, a study conducted in T-cells demonstrated that Src activation is necessary for the induction of SRF and transcriptional regulation of target genes (Katsch et al., 2012). Further studies are necessary to examine the Src-mediated transcriptional regulation in myofibroblasts.
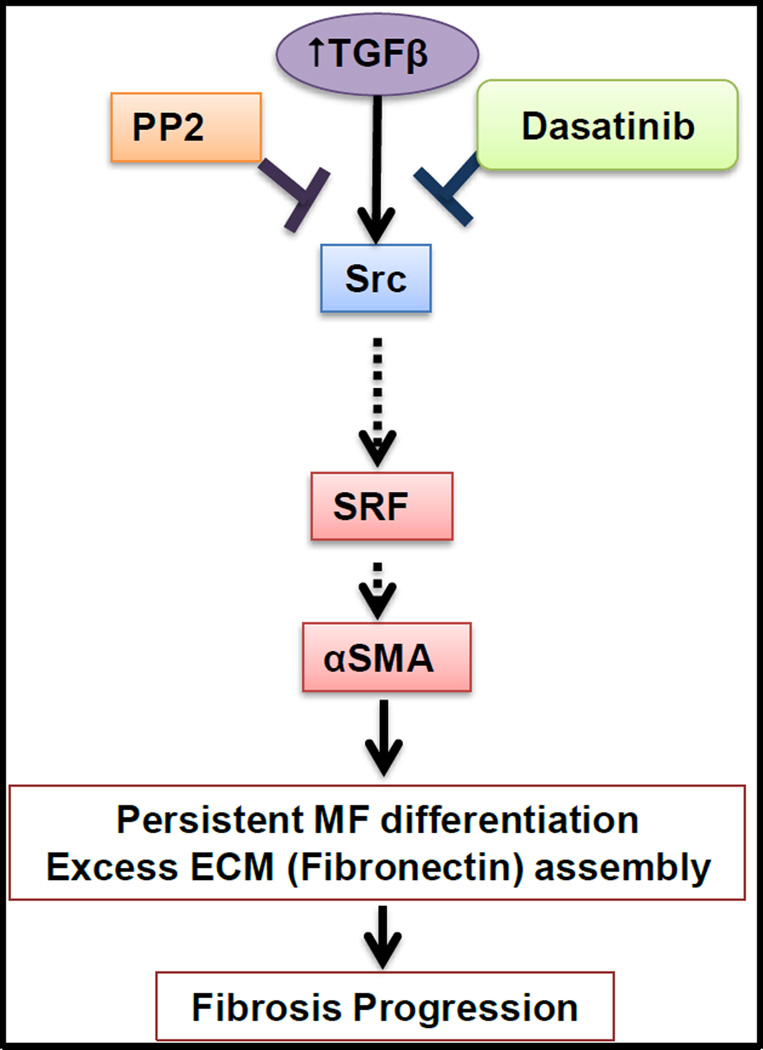
Collectively, the present study demonstrates that dasatinib is a potent modulator of TGFβ-induced myofibroblast differentiation and ECM accumulation in both NIH 3T3 and HFLFs. Mechanistically, similar to PP2, dasatinib attenuates αSMA synthesis, in part, through inhibition of Src kinase signaling and the transcription factor SRF (Fig. 6). These findings suggest that Src kinases might be a promising target of dasatinib. Thus, dasatinib could be a potential anti-fibrotic therapeutic strategy in IPF and fibrotic diseases.
Fig. 6.
A schematic representation of the working hypothesis that dasatinib and PP2 inhibit TGFβ-induced αSMA expression and fibronectin assembly, in part via regulation of Src and SRF, thereby attenuating myofibroblast differentiation.
Supplementary Material
Acknowledgements
Funds were provided by the National Institutes of Health grant (R01HL103952), University of Georgia Research Foundation and Wilson Pharmacy Foundation to PRS. MA was supported by a pre-doctoral fellowship from American Heart Association (13PRE17100070). This material is the result of work supported with resources and the use of facilities at the Charlie Norwood VAMC, Augusta, GA. The funders had no role in the study design, data collection, analysis and decision to publish. Preparation of the manuscript and the contents do not represent the views of the Department of Veterans Affairs or the United States Government.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of interest: The authors have declared that no conflicts of interest exist
References
- Abdalla M, Goc A, Segar L, Somanath PR. Akt1 mediates alpha-smooth muscle actin expression and myofibroblast differentiation via myocardin and serum response factor. J Biol Chem. 2013;288(46):33483–33493. doi: 10.1074/jbc.M113.504290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aono Y, Nishioka Y, Inayama M, Ugai M, Kishi J, Uehara H, et al. Imatinib as a novel antifibrotic agent in bleomycin-induced pulmonary fibrosis in mice. Am J Respir Crit Care Med. 2005;171(11):1279–1285. doi: 10.1164/rccm.200404-531OC. [DOI] [PubMed] [Google Scholar]
- Atfi A, Drobetsky E, Boissonneault M, Chapdelaine A, Chevalier S. Transforming growth factor beta down-regulates Src family protein tyrosine kinase signaling pathways. J Biol Chem. 1994;269(48):30688–30693. [PubMed] [Google Scholar]
- Daniels CE, Lasky JA, Limper AH, Mieras K, Gabor E, Schroeder DR, et al. Imatinib treatment for idiopathic pulmonary fibrosis: Randomized placebo-controlled trial results. Am J Respir Crit Care Med. 2010;181(6):604–610. doi: 10.1164/rccm.200906-0964OC. [DOI] [PubMed] [Google Scholar]
- Daniels CE, Wilkes MC, Edens M, Kottom TJ, Murphy SJ, Limper AH, et al. Imatinib mesylate inhibits the profibrogenic activity of TGF-beta and prevents bleomycin-mediated lung fibrosis. J Clin Invest. 2004;114(9):1308–1316. doi: 10.1172/JCI19603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuda K, Kawata S, Tamura S, Matsuda Y, Inui Y, Igura T, et al. Altered regulation of Src tyrosine kinase by transforming growth factor beta1 in a human hepatoma cell line. Hepatology. 1998;28(3):796–804. doi: 10.1002/hep.510280329. [DOI] [PubMed] [Google Scholar]
- Goc A, Choudhary M, Byzova TV, Somanath PR. TGFβ- and bleomycin-induced extracellular matrix synthesis is mediated through Akt and mammalian target of rapamycin (mTOR) J Cell Physiol. 2011;226(11):3004–3013. doi: 10.1002/jcp.22648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gressner AM, Weiskirchen R. Modern pathogenetic concepts of liver fibrosis suggest stellate cells and TGF-beta as major players and therapeutic targets. J Cell Mol Med. 2006;10(1):76–99. doi: 10.1111/j.1582-4934.2006.tb00292.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinz B, Gabbiani G. Fibrosis: recent advances in myofibroblast biology and new therapeutic perspectives. F1000 biology reports. 2010;2:78. doi: 10.3410/B2-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinz B, Phan SH, Thannickal VJ, Prunotto M, Desmoulière A, Varga J, et al. Recent developments in myofibroblast biology: paradigms for connective tissue remodeling. Am J Pathol. 2012;180(4):1340–1355. doi: 10.1016/j.ajpath.2012.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hostettler KE, Zhong J, Papakonstantinou E, Karakiulakis G, Tamm M, Seidel P, et al. Anti-fibrotic effects of nintedanib in lung fibroblasts derived from patients with idiopathic pulmonary fibrosis. Respir Res. 2014;15(1):157. doi: 10.1186/s12931-014-0157-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu M, Che P, Han X, Cai GQ, Liu G, Antony V, et al. Therapeutic targeting of SRC kinase in myofibroblast differentiation and pulmonary fibrosis. J Pharmacol Exp Ther. 2014;351(1):87–95. doi: 10.1124/jpet.114.216044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kantarjian H, Jabbour E, Grimley J, Kirkpatrick P. Dasatinib. Nat Rev Drug Discov. 2006;5(9):717–718. doi: 10.1038/nrd2135. [DOI] [PubMed] [Google Scholar]
- Katsch K, de Jong SJ, Albrecht JC, Steger J, Genth H, Posern G, et al. Actin-dependent activation of serum response factor in T cells by the viral oncoprotein tip. Cell Commun Signal. 2012;10(1):5. doi: 10.1186/1478-811X-10-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HP, Kim TY, Lee MS, Jong HS, Lee JW, Bang YJ. TGF-beta1-mediated activations of c-Src and Rac1 modulate levels of cyclins and p27(Kip1) CDK inhibitor in hepatoma cells replated on fibronectin. Biochim Biophys Acta. 2005;1743(1–2):151–161. doi: 10.1016/j.bbamcr.2004.09.014. [DOI] [PubMed] [Google Scholar]
- Li M, Abdollahi A, Gröne HJ, Lipson KE, Belka C, Huber PE. Late treatment with imatinib mesylate ameliorates radiation-induced lung fibrosis in a mouse model. Radiat Oncol. 2009;4:66. doi: 10.1186/1748-717X-4-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin YL, Meng Y, Jiang W, Roux B. Explaining why Gleevec is a specific and potent inhibitor of Abl kinase. Proc Natl Acad Sci U S A. 2013;110(5):1664–1669. doi: 10.1073/pnas.1214330110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manganini M, Maier JA. Transforming growth factor beta2 inhibition of hepatocyte growth factor-induced endothelial proliferation and migration. Oncogene. 2000;19(1):124–133. doi: 10.1038/sj.onc.1203225. [DOI] [PubMed] [Google Scholar]
- Mishra R, Zhu L, Eckert RL, Simonson MS. TGF-beta-regulated collagen type I accumulation: role of Src-based signals. Am J Physiol Cell Physiol. 2007;292(4):C1361–C1369. doi: 10.1152/ajpcell.00370.2006. [DOI] [PubMed] [Google Scholar]
- Montero JC, Seoane S, Ocaña A, Pandiella A. Inhibition of SRC family kinases and receptor tyrosine kinases by dasatinib: possible combinations in solid tumors. Clin Cancer Res. 2011;17(17):5546–5552. doi: 10.1158/1078-0432.CCR-10-2616. [DOI] [PubMed] [Google Scholar]
- Okutani D, Lodyga M, Han B, Liu M. Src protein tyrosine kinase family and acute inflammatory responses. Am J Physiol Lung Cell Mol Physiol. 2006;291(2):L129–L141. doi: 10.1152/ajplung.00261.2005. [DOI] [PubMed] [Google Scholar]
- Park SS, Eom YW, Kim EH, Lee JH, Min DS, Kim S, et al. Involvement of c-Src kinase in the regulation of TGF-beta1-induced apoptosis. Oncogene. 2004;23(37):6272–6281. doi: 10.1038/sj.onc.1207856. [DOI] [PubMed] [Google Scholar]
- Pechkovsky DV, Scaffidi AK, Hackett TL, Ballard J, Shaheen F, Thompson PJ, et al. Transforming growth factor beta1 induces alphavbeta3 integrin expression in human lung fibroblasts via a beta3 integrin-, c-Src-, and p38 MAPK-dependent pathway. J Biol Chem. 2008;283(19):12898–12908. doi: 10.1074/jbc.M708226200. [DOI] [PubMed] [Google Scholar]
- Rice AB, Moomaw CR, Morgan DL, Bonner JC. Specific inhibitors of platelet-derived growth factor or epidermal growth factor receptor tyrosine kinase reduce pulmonary fibrosis in rats. Am J Pathol. 1999;155(1):213–221. doi: 10.1016/S0002-9440(10)65115-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richeldi L, du Bois RM, Raghu G, Azuma A, Brown KK, Costabel U, et al. Efficacy and safety of nintedanib in idiopathic pulmonary fibrosis. N Engl J Med. 2014;370(22):2071–2082. doi: 10.1056/NEJMoa1402584. [DOI] [PubMed] [Google Scholar]
- Roskoski R. Src protein-tyrosine kinase structure, mechanism, and small molecule inhibitors. Pharmacol Res. 2015;94:9–25. doi: 10.1016/j.phrs.2015.01.003. [DOI] [PubMed] [Google Scholar]
- Schlaepfer DD, Hunter T. Focal adhesion kinase overexpression enhances ras-dependent integrin signaling to ERK2/mitogen-activated protein kinase through interactions with and activation of c-Src. J Biol Chem. 1997;272(20):13189–13195. doi: 10.1074/jbc.272.20.13189. [DOI] [PubMed] [Google Scholar]
- Seeliger MA, Nagar B, Frank F, Cao X, Henderson MN, Kuriyan J. c-Src binds to the cancer drug imatinib with an inactive Abl/c-Kit conformation and a distributed thermodynamic penalty. Structure. 2007;15(3):299–311. doi: 10.1016/j.str.2007.01.015. [DOI] [PubMed] [Google Scholar]
- Seeliger MA, Ranjitkar P, Kasap C, Shan Y, Shaw DE, Shah NP, et al. Equally potent inhibition of c-Src and Abl by compounds that recognize inactive kinase conformations. Cancer Res. 2009;69(6):2384–2392. doi: 10.1158/0008-5472.CAN-08-3953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skhirtladze C, Distler O, Dees C, Akhmetshina A, Busch N, Venalis P, et al. Src kinases in systemic sclerosis: central roles in fibroblast activation and in skin fibrosis. Arthritis Rheum. 2008;58(5):1475–1484. doi: 10.1002/art.23436. [DOI] [PubMed] [Google Scholar]
- Somanath PR, Kandel ES, Hay N, Byzova TV. Akt1 signaling regulates integrin activation, matrix recognition, and fibronectin assembly. The Journal of biological chemistry. 2007;282(31):22964–22976. doi: 10.1074/jbc.M700241200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thannickal VJ, Lee DY, White ES, Cui Z, Larios JM, Chacon R, et al. Myofibroblast differentiation by transforming growth factor-beta1 is dependent on cell adhesion and integrin signaling via focal adhesion kinase. J Biol Chem. 2003;278(14):12384–12389. doi: 10.1074/jbc.M208544200. [DOI] [PubMed] [Google Scholar]
- Tomasek JJ, Gabbiani G, Hinz B, Chaponnier C, Brown RA. Myofibroblasts and mechano-regulation of connective tissue remodelling. Nature reviews. Molecular cell biology. 2002;3(5):349–363. doi: 10.1038/nrm809. [DOI] [PubMed] [Google Scholar]
- Vittal R, Horowitz JC, Moore BB, Zhang H, Martinez FJ, Toews GB, et al. Modulation of prosurvival signaling in fibroblasts by a protein kinase inhibitor protects against fibrotic tissue injury. Am J Pathol. 2005;166(2):367–375. doi: 10.1016/S0002-9440(10)62260-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vittal R, Zhang H, Han MK, Moore BB, Horowitz JC, Thannickal VJ. Effects of the protein kinase inhibitor, imatinib mesylate, on epithelial/mesenchymal phenotypes: implications for treatment of fibrotic diseases. J Pharmacol Exp Ther. 2007;321(1):35–44. doi: 10.1124/jpet.106.113407. [DOI] [PubMed] [Google Scholar]
- Wollin L, Maillet I, Quesniaux V, Holweg A, Ryffel B. Antifibrotic and anti-inflammatory activity of the tyrosine kinase inhibitor nintedanib in experimental models of lung fibrosis. J Pharmacol Exp Ther. 2014;349(2):209–220. doi: 10.1124/jpet.113.208223. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.