Abstract:
Developing new strategies to improve patient safety and risk reduction is fundamental to hospital and patient success. Currently, there is a tendency in hospital safety management to focus solely on human error rather than organizational and educational causes that contribute to medical accidents. Although health care providers are the primary safety systems in medical facilities, there must be a more global, perhaps automated, approach using modern technology to prevent or reduce medical mishaps. Herein, we present an oxygenation failure with root cause analysis that prompted a new oxygenation safety algorithm and multi-service training initiative.
Keywords: safety, oxygenation failure, root cause analysis, algorithm
Several studies have suggested that at least 5%–10% of hospital admissions will suffer some form of adverse event (1–3). Similarly, procedures involving cardiopulmonary bypass (CPB), although considered routine, are not without risk. In fact, CPB procedures have a relatively high risk to time ratio because minimal time is spent in the operating room compared with the potential risk. With the use of CPB, the potential for injury can be great. This makes recognition of atypical events paramount in determining their etiology and successful methods of management. Once an atypical event, such as an oxygenation failure, is identified, a root cause analysis (RCA) can be a useful tool to explore strategies of prevention. The RCA process involves the gathering of all those present during an event to analyze common factors and contributing actions that lead to medical mishaps. The ultimate goal of an RCA is to prevent reoccurrence.
The safe conduct of perfusion depends most heavily on a well-trained, attentive perfusionist; however, this alone will not eliminate risk or guarantee success (4,5). Successful risk reduction is seeded in multi-disciplinary cardiac teams and organizations with a high level of communication that have a shared vision of patient safety, quality assurance, and risk management (6).
CASE DESCRIPTION
A 45-year-old woman with anxiety disorder developed chest pain that was associated with nausea and vomiting after a stressful event. She was taken to an outlying hospital and treated with nitroglycerin and received a 12-lead electrocardiogram (EKG). The 12-lead EKG showed T-wave inversions that subsequently prompted transfer to a medical facility with cardiac catheterization capability. She further showed a small non-Q-wave infarction of the anterior myocardium with troponin (T) = 0.87 ng/mL and creatinine kinase = 280 units/L. All other laboratory tests were within normal limits. A cardiac catheterization revealed preserved left ventricular function, with a 90% lesion of the left anterior descending (LAD) artery. She was given 300 mg Plavix (Bristol-Myers Squibb/Sanofi Pharmaceuticals Partnership, New York, NY) by mouth and placed on an Integrilin (COR Therapeutics, South San Francisco, CA, and Key Pharmaceuticals, Kenilworth NJ) drip for transfer to a cardiac operative facility.
On arrival to the operative facility, she consented for coronary artery bypass graft surgery to be conducted by taking down the left internal mammary artery (LIMA) in a pedicle and performing an end-to-side anastomosis to the LAD coronary artery using CPB.
CASE MANAGEMENT
The extracorporeal circuit was prepared in a routine fashion according to the pre-bypass checklist and primed with 2000 mL of Plasmalyte A (Baxter Healthcare, Deer-field, IL), 2000 USP bovine heparin, and 12.5 g mannitol. After induction of satisfactory general anesthesia and insertion of monitoring lines, the patient was prepared and draped in a routine sterile fashion. Standard cannulation was performed with a 7.0 Softflow cannula (Terumo Cardiovascular, Ann Arbor, MI) accessing the ascending aorta and a 29/37 three-stage cannula (Edwards Lifesciences, Irvine, CA) inserted through the right atrial appendage for venous drainage. After heparinization and verification of an activated clotting of 610 seconds, CPB was initiated.
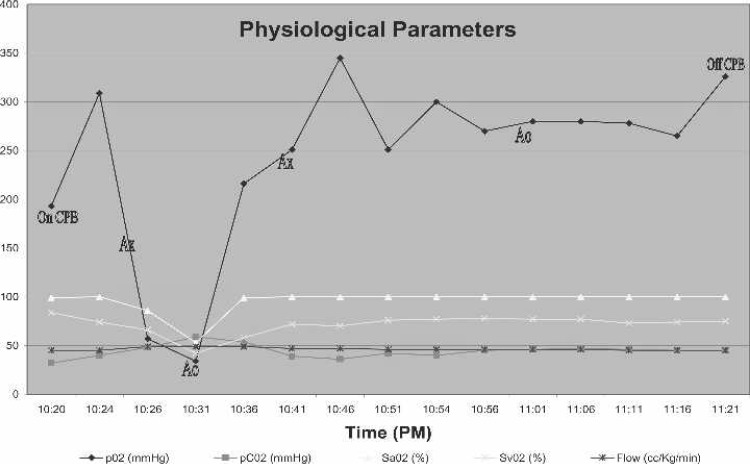
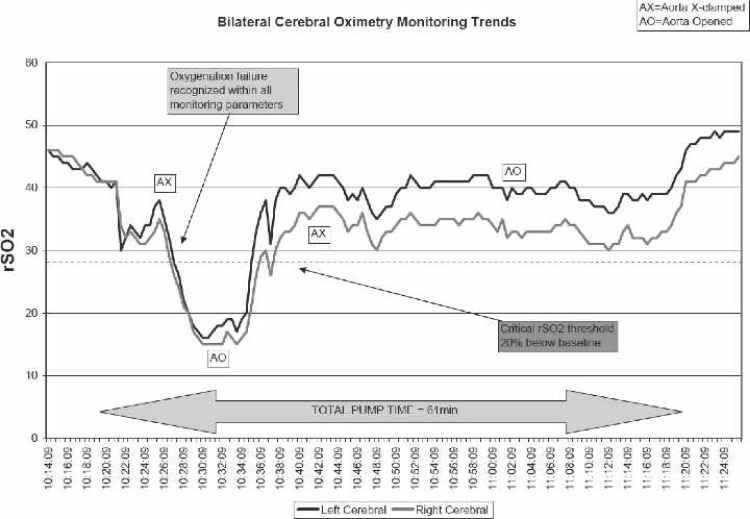
Approximately 5 minutes after initiating bypass, there was a precipitous drop in PaO2, SaO2, and SvO2, and a rise in CO2 as indicated by continuous in-line blood gas monitoring (CDI 500; Terumo Cardiovascular, Ann Arbor, MI; Figure 1). In addition, there was a significant drop in rSO2 as indicated by a cerebral oximetry monitor (INVOS, 5100B; Somanetics, Troy, MI; Figure 2). Both the CDI 500 and INVOS monitors were alarming during the critical oxygenation event. The blender gas line connections were assessed at the anesthesia/perfusion boom and found to be intact. Furthermore, the blender was not alarming to indicate a pressure differential and possible disconnection. Gas flow to the oxygenator was not assessed by tactile evaluation or other method, although the blender was advanced to 6 L/min of flow. After discussion with anesthesia and the surgeon, a decision was made to transfer another heart/lung machine into the surgical suite to use its blender apparatus with the existing oxygenator. During this time, the aortic cross-clamp was removed, followed by internal cardiac massage and pulmonary ventilation. After transfer of a different heart/lung machine and use of its blender apparatus, an immediate return of pO2, SaO2, SvO2, CO2, and rSO2 to a physiologic state was established, which allowed resumption of the procedure. The aortic cross-clamp was re-applied, and the LIMA was grafted to the LAD. The remainder of the case was uneventful, with a total CPB time of 61 minutes and total aortic cross-clamp time of 26 minutes.
Figure 1.
Graphical representation of physiologic parameters relevant to time course of events. Ax, aorta cross-clamped; Ao, aorta opened.
Figure 2.
Cerebral oximetry during CPB.
The postoperative course was uneventful, with no identifiable neurocognitive sequelae. The patient was discharged on day 4 after surgery.
DISCUSSION
Because of the course of events, a second perfusionist was contacted to evaluate the equipment and situation. The blender apparatus in question was tested and found to be operational. Gas flow was assessed at the oxygenator through tactile evaluation with the vaporizer “on” and in the “off” positions. Furthermore, the blender was removed from the heart/lung machine and transferred to the biomedical department for bench top evaluation. The biomedical evaluation found the blender to be working within normal tolerances. This particular blender had preventative maintenance conducted 11 months before and was returned as satisfactory.
On ruling out the blender, the only other equipment in-line between the blender and oxygenator was a Forane vaporizer (Tec 7; Datex-Ohmeda, Madison, WI). Although the vaporizer was working properly, it can leak gas if either improperly seated on the holder or if the fill cap is not properly tightened. The vaporizer can be bypassed by turning to the “off” position.
While gas flow to the oxygenator was assessed by tactile evaluation with the vaporizer in the “on” and “off” positions according to our pre-bypass checklist before initiating CPB, we speculated that the vaporizer was likely the source that led to the near-miss oxygenation failure and RCA. After the RCA and analyzing the facts associated with the near-miss, the critical oxygenation event was replicated by tilting the vaporizer in such a fashion that gas flow was able to escape without reaching the oxygenator while the blender flow remained static at 6 L/min.
The RCA identified several areas of concern relevant to the oxygenation failure episode: gas flow was not assessed at the oxygenator; the vaporizer was not turned off; crucial time was spent transferring an additional heart/lung machine to the operative suite; and the anesthesia gas flow meter was not used.
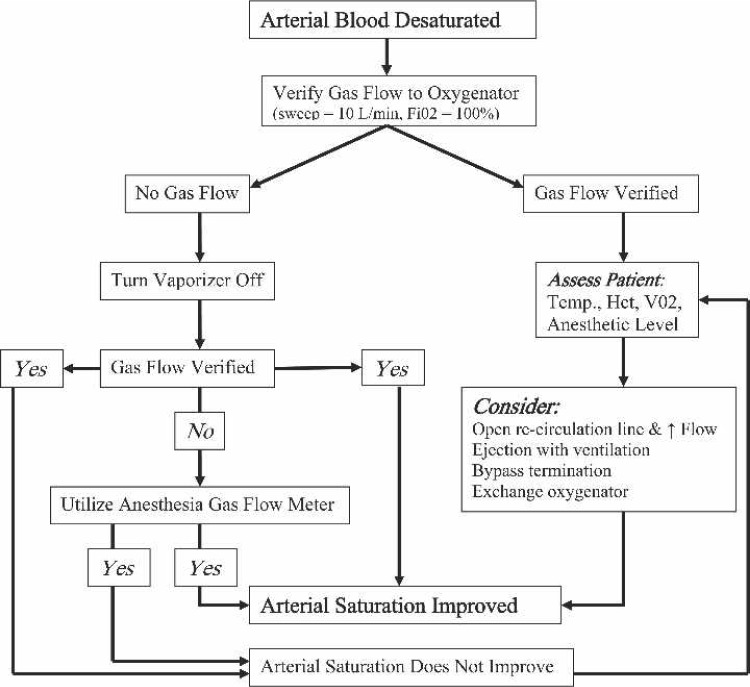
The likelihood of a much different patient outcome is easily imagined had the time course been different or if the source of the problem had been the oxygenator (7–10). This prompted the development of an oxygenation algorithm (Figure 3) and training initiative. A small, direct interview type survey was further conducted, and found that required training should not be isolated to a single individual or individuals involved in this near miss (11). Thirty cardiac personnel including both perfusionists and cardiac anesthesiologists from four separate facilities were queried as to how they would respond to a similar event, and in particular, if they were aware of a gas flow meter on the anesthesia machine that could be attached to a CPB oxygenator. Approximately 93% of those surveyed were unaware that anesthesia machines contain an oxygen flow meter that can be readily attached to any CPB oxygenator. In the surgical suite where this near miss occurred, the anesthesia oxygen flow meter was ∼4 ft from the heart/lung machine, and connections between devices would have taken <20 seconds (Figure 4).
Figure 3.
Oxygenation algorithm placed on all heart/lung machines.
Figure 4.

Anesthesia gas flow meter attached to CPB oxygenator.
Although our pre-bypass checklist had contained a parameter involving a back-up oxygen bottle, this is somewhat impractical because we are often tasked to apply CPB in non-dedicated cardiac suites in an emergent fashion. All of our anesthesia machines contain a back-up oxygen flow meter that is rarely, if ever, used with patients that are intubated. When a non-dedicated cardiac suite is used, the perfusionist must identify its location and a method of attachment.
CONCLUSION
A collaborative fundamental understanding of the basic resources needed to perform routine tasks by all team members will not only improve continuity but may also reduce liability and medical mishaps. Although an RCA is an excellent tool to identify and prevent medical mishaps at the local level, there should be a more global approach using modern technology to communicate and improve patient safety. The continued process of advancing patient safety and risk reduction measures in health care are the foundation of successful teams, facilities, and organizations.
REFERENCES
- 1.Graves K.. Perfusion safety in Europe: Managing risks, learning from mistakes. Perfusion. 2005;20:209–15. [DOI] [PubMed] [Google Scholar]
- 2.Brennan TA, Leape LL, Laird NM, et al. Incidence of adverse events and negligence in hospitalized patients. Results of the Harvard Medical Practice Study I. N Engl J Med. 1991;324:370–76. [DOI] [PubMed] [Google Scholar]
- 3.Leape LL, Brennan TA, Laird NM, et al. The nature of adverse events in hospitalized patients. Results of the Harvard Medical Practice Study II. N Engl J Med. 1991;324:377–84. [DOI] [PubMed] [Google Scholar]
- 4.Palanzo DA.. Perfusion safety: Past, present, and future. J Cardiothorac Vasc Anesth. 1997;11:383–90. [DOI] [PubMed] [Google Scholar]
- 5.Palanzo DA.. Perfusion safety: Defining the problem. Perfusion. 2005;20:195–203. [DOI] [PubMed] [Google Scholar]
- 6.Svenmaker S, Haggmark S, Jansson E, Lindholm MA, Aberg T.. Quality assurance in clinical perfusion. Euro J Card Thor Surg. 1998;14:409–14. [DOI] [PubMed] [Google Scholar]
- 7.Stammers AH, Majek BL.. An update on perfusion safety: Does the type of perfusion practice affect the rate of incidence related to cardiopulmonary bypass. Perfusion. 2001;16:189–98. [DOI] [PubMed] [Google Scholar]
- 8.Kriewall TJ.. Manufacturers approach in the development of intelligent multilevel safety systems to assist perfusionists during cardiopulmonary bypass. Perfusion. 2005;20:227–32. [DOI] [PubMed] [Google Scholar]
- 9.Fisher AR.. The incidence and cause of emergent oxygenator changeover. Perfusion. 1999;14:207–12. [DOI] [PubMed] [Google Scholar]
- 10.Wierenga EJ.. Safety: A manufacturer’s perspective. Perfusion. 1997;12:229–31. [DOI] [PubMed] [Google Scholar]
- 11.Kurusz M.. Lessons from perfusion surveys. Perfusion. 1997;12:221–7. [DOI] [PubMed] [Google Scholar]