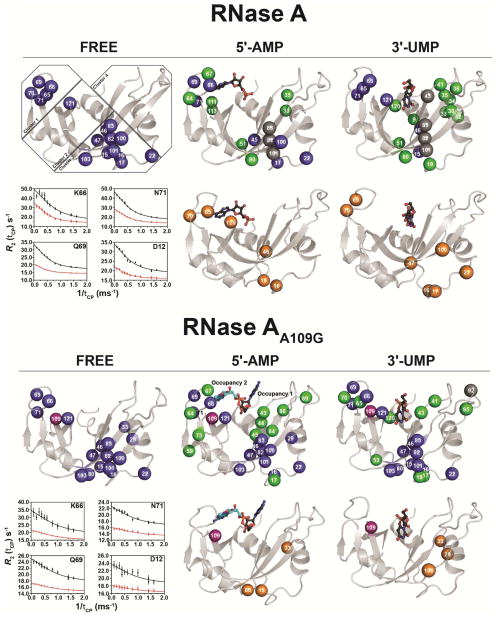
Figure 3.
Effects of 5′-AMP and 3′-UMP binding on the millisecond dynamics of WT RNase A and mutant A109G. Millisecond time scale (ms) conformational exchange experienced by apo, 5′-AMP- and 3′-UMP-bound forms of WT RNase A and mutant A109G. Motions were probed by 15N-CPMG relaxation dispersion experiments at 500 MHz and 800 MHz (298 K). Residues were considered for further analysis only if the difference in measured R2 (1/Tcp) values at fast (Tcp = 0.625 ms) and slow (Tcp = 10 ms) pulsing rates was greater than 2 s−1, similar to previous reports (Cole and Loria, 2002; Doucet et al., 2009; Gagne et al., 2012). Blue spheres: residues showing 15N-CPMG dispersion profiles with ΔR2 (1/Tcp) > 2 s−1; orange spheres: residues showing no conformational exchange relative to the apo form (i.e., dampened ms dynamics upon ligand binding) or outside of the 15N-CPMG time frame; green spheres: residues gaining conformational exchange [ΔR2 (1/Tcp) > 2] upon ligand binding; gray spheres: assigned residues in the apo form that cannot be assigned in the ligand-bound form due to line broadening; purple spheres: position 109. Residues are highlighted on the 3D structure of WT RNase A (apo form: PDB 7RSA; 3′-UMP-bound: PDB 1O0N; 5′-AMP-bound: PDB 1Z6S) and mutant A109G (apo form: PDB 4WYN; 3′-UMP-bound: PDB 4WYZ; 5′-AMP-bound: PDB 4WYP). Insets show representative dispersion curves acquired at 500 MHz (red) and 800 MHz (black) for a subset of loop 4 (cluster 1) in apo forms of WT and A109G. Residue clusters are identified according to (Gagne et al., 2012).