Figure 1.
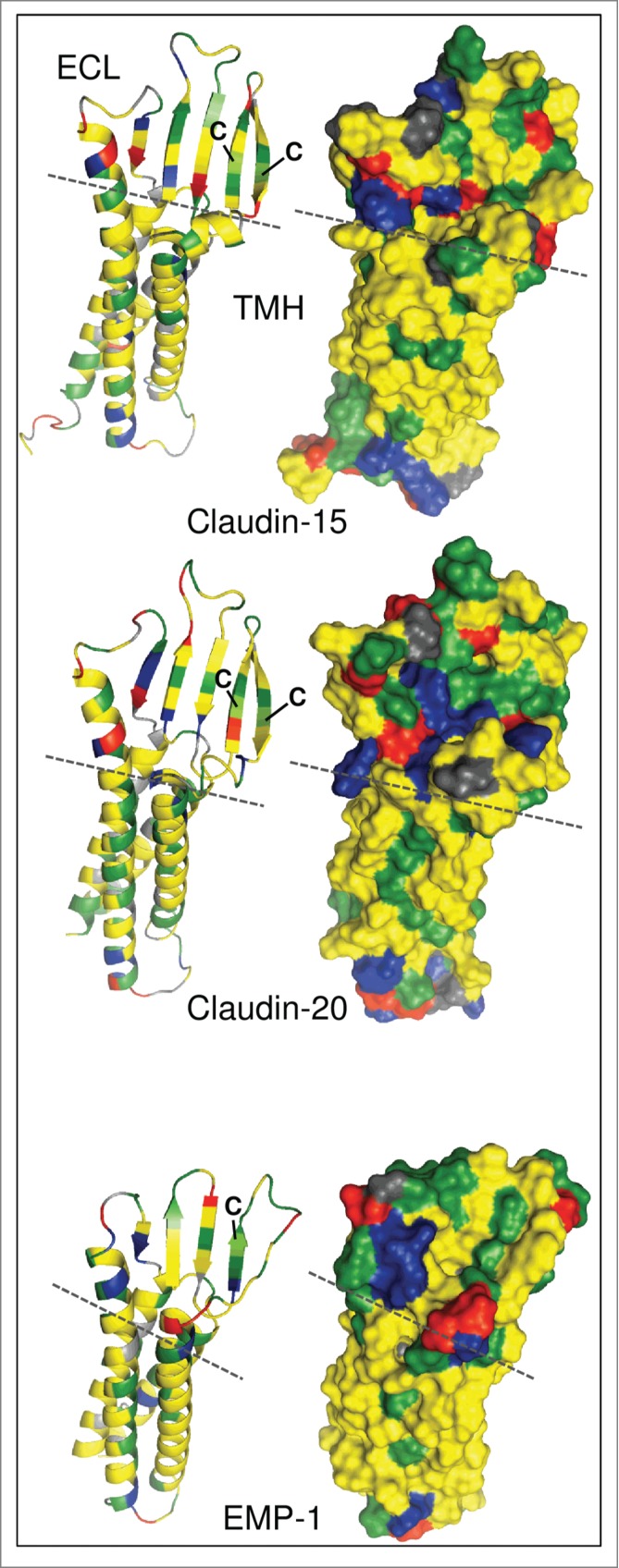
Molecular modeling of human claudin-15, claudin-20 and epithelial membrane protein 1 (EMP-1). Left: Ribbon models showing secondary structural elements. Right: Surface models showing exposed amino acid (aa) residues. Modeling was performed using I-TASSER.9 without additional restraints and excluding C-terminal cytosolic aa. Claudin-20 and EMP-1 models were aligned to claudin-15 using PyMOL (http://www.pymol.org). Claudin-15: aa 1–193, Claudin-20: aa 1–186, EMP-1: aa 1–154. Green, hydrophilic aa; yellow, hydrophobic aa; blue, positively charged aa; red, negatively charged aa; gray, glycine, proline; dashed line, border between transmembrane helices (TMH) and extracellular loops (ECL); C, conserved cysteines in the first ECL.
