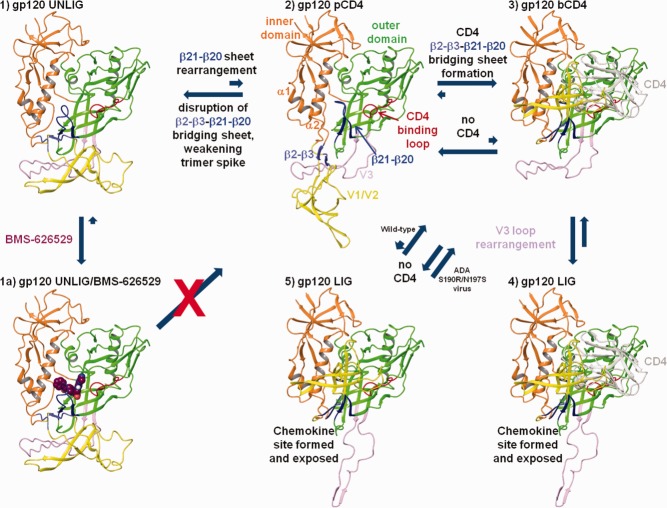
Figure 10.
Proposed stepwise pathway for HIV-1 gp-120 attachment, CD4-independent attachment, and inhibition by attachment inhibitors. 1. In the gp120 UNLIG state the bridging sheet is ordered β2–β3-β21–β20 and directs the V1/V2 loops to pack against the V3 loop where they collectively form the quaternary structure of the trimer spike crown. In this state the β21–β20 sheet is misfolded for optimal CD4 binding. Note: the β21–β20 sheet is in equilibrium between nonCD4 and CD4 binding conformations with the nonCD4 binding conformations being the dominant form. 2. In the gp120 pre-CD4 state (pCD4) the β2–β3-β21–β20 bridging sheet is disrupted and the β21–β20 sheet is in equilibrium between nonCD4 and CD4 binding conformations. Based on SAXS data, this is likely to be the predominant form of the gp120 monomer in solution. Note that in the absence of CD4 (1), (2), (3), and (5) are in equilibrium but due to tertiary and quaternary structure involving the bridging sheet and the V1/V2/V3 loops (1) is far more prevalent; however, in the lab-adapted ADA S190R/N197S virus, the population of (2), (3) and (5) are elevated, leading to CD4-independent attachment via (5). 3. In the CD4-bound state (bCD4) CD4 stabilizes the β21–β20 sheet in the CD4 binding conformation and the re-ordered bridging sheet forms (β3–β2-β21–β20) moving the V1/V2 loops near CD4 domains I and II. 4. In the gp120 LIG state the V3 loop has opened to complete the formations and exposure of the chemokine binding site. 1a. BMS-626529 and related AIs are predicted to bind within the structurally conserved region in the outer domain of gp120 UNLIG (1) state. AIs binding within this pocket stabilize the UNLIG state by blocking the β21–β20 sheet from folding into a CD4-binding conformation and occupy the space next to the CD4 binding loop required for CD4:F43 binding, which blocks CD4 binding and the formation of the gp120 pCD4 state and all downstream events.