CASE PRESENTATION
Brief History of the Present Illness
A 13-month-old female from New Mexico presented to a local emergency department with 5 days of fever, diarrhea, nasal congestion, and cough, 2 days of jaundice, and 1 day of an erythematous papular rash on her trunk, arms, and groin. Initial laboratory studies revealed severe macrocytic anemia, reticulocytosis, and a positive direct Coombs test. She was hospitalized at a local facility for management of severe hemolytic anemia. Hemolysis was persistent despite daily blood transfusions, intravenous immune globulin (IVIG), and high-dose methylprednisolone. A bone marrow aspirate revealed tri-lineage marrow with reactive erythroid hyperplasia and absence of blasts. Her rash subsequently progressed from papules to ulcerated, crusted lesions. A lesional skin biopsy revealed intranuclear inclusion bodies typical of herpesviridae; therefore, acyclovir was initiated 3 weeks into her illness. She later developed encephalopathy characterized by loss of truncal tone and speech, decreased alertness, and difficulty managing oral secretions. After 4 weeks of progressive illness, she was transferred to our facility for further management.
Past Medical History
The patient was previously healthy except for frequent upper respiratory infections; she had been diagnosed with “viral-induced wheeze,” which was treated with inhaled albuterol as needed. Growth and development were normal. She received all immunizations as per the Advisory Committee on Immunization Practices recommended schedule, including the rotavirus series. The following immunizations were administered 3 weeks before her onset of illness: Haemophilus influenzae type b, hepatitis A, measles, mumps, and rubella, pneumococcal conjugate, and varicella.
Key Medications
Upon transfer, she was receiving acyclovir, methylprednisolone, and total parenteral nutrition.
Family History
An 8-year-old sister developed H influenzae (serotype unknown) meningitis at age 12 months, and she recovered fully except for hearing loss. She had been vaccinated against H influenzae type b before onset of meningitis.
Epidemiological History
The patient and her family had recently camped recreationally in Texas and Florida, but they had not traveled outside of the United States. There were no known animal or unusual environmental exposures, insect bites, or sick contacts.
Physical Examination
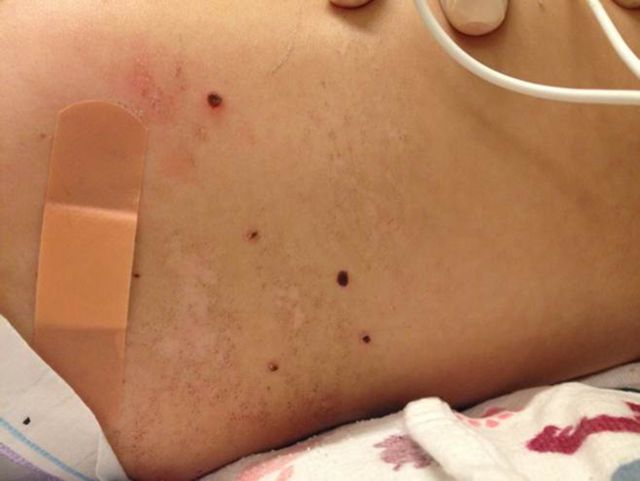
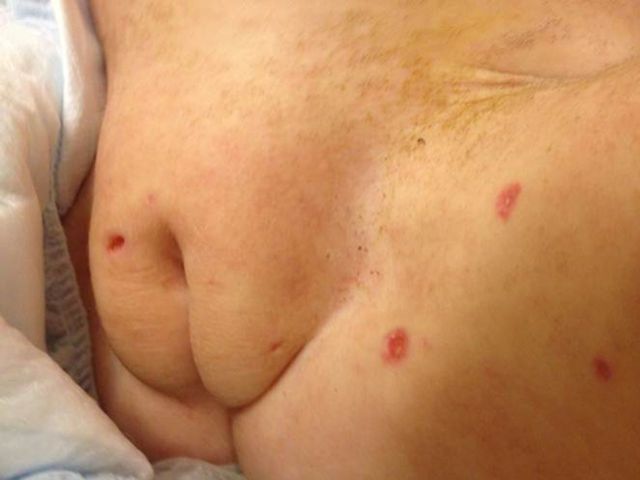
On admission, temperature was 37.1°C, pulse 123 beats per minute, blood pressure 134/76 mmHg, and respirations 33 breaths per minute, with oxygen saturation 100% on 2 liters per minute of supplemental oxygen via nasal cannula. The patient was awake but listless and minimally responsive to stimuli. Pupils were equal and reactive; she did not track the examiner. There were no oral lesions, but white nonpurulent oral secretions were present. Her neck was deviated to the right in a dystonic position. She had no respiratory distress, but rhonchi were present on lung auscultation. Liver and spleen were palpable 1–2 cm below the respective costal margins. Generalized weakness, head lag, and poor truncal tone were present. She moved all extremities nonpurposefully. Deep tendon reflexes were absent. A rash consisting of “punched-out” ulcerated lesions with overlying serosanguineous crust, 2–5 mm in diameter, was present on the trunk, genitalia, and proximal extremities (Figures 1 and 2).
Figure 1.
Rash consisting of discrete crusted lesions on the trunk.
Figure 2.
Rash consisting of “punched-out” ulcerated lesions on the genitalia.
Initial Laboratory and Radiographic Studies
The total leukocyte count was 12 400/µL, with 77% neutrophils, 5% immature neutrophils, 7% lymphocytes, and 11% monocytes; hematocrit 21.8% (reference range [ref.] 30–41), hemoglobin 7.7 g/dL (ref. 9.5–14), and platelets 346 000/µL. Serum albumin was 3.1 g/dL (ref. 3.4–4.2), aspartate aminotransferase was 78 U/L (ref. 20–60), alanine aminotransferase was 87 U/L (ref. 5–45), and total bilirubin was 1.9 mg/dL (ref. 0.2–1.2). The remainder of a comprehensive metabolic panel was normal. Serum lactate dehydrogenase was 2586 U/L (ref. 500–920), and ferritin was 1310 ng/mL (ref. 10–60).
Cerebrospinal fluid (CSF) cytology revealed 0 leukocytes, 19 erythrocytes, glucose 69 mg/dL (ref. 40–75), and protein <10 mg/dL (ref. 12–60). Gram stain and culture of the CSF were negative.
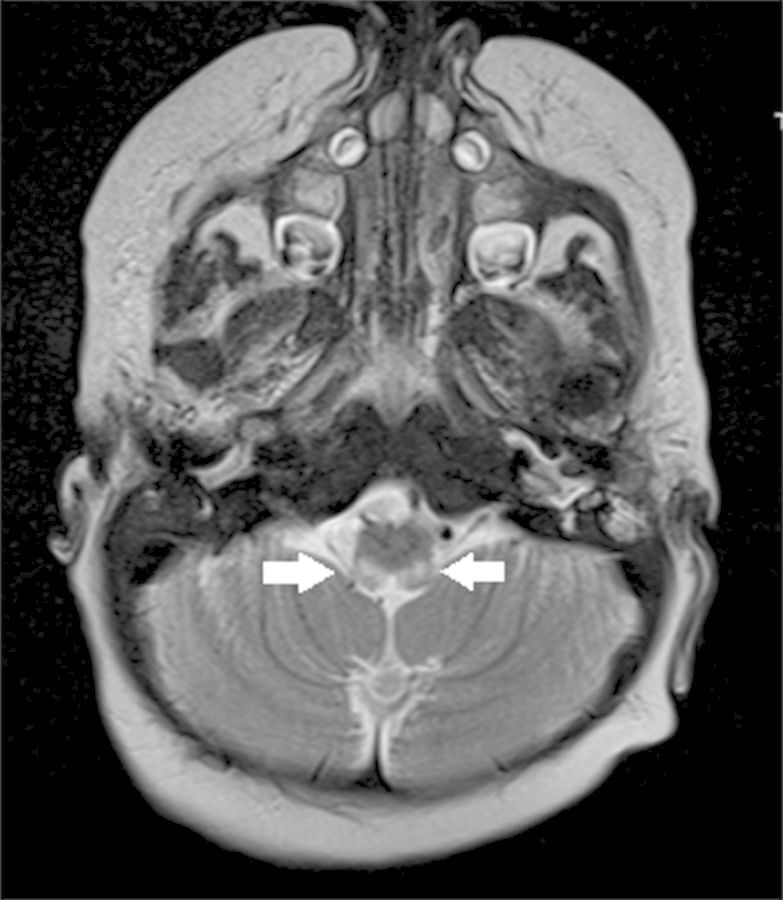
A magnetic resonance image of the brain showed symmetric increased T2 signal involving the brachium pontis bilaterally, extending inferiorly to involve the dorsal medulla and upper cervical spinal cord (Figure 3).
Figure 3.
Magnetic resonance image of the brain with symmetric increased T2 signal involving the dorsal medulla and upper cervical spinal cord.
Clinical Course Before Diagnosis
Due to rapidly declining mental status, the patient was intubated soon after transfer. Severe hemolytic anemia persisted, requiring numerous blood transfusions and treatment with additional methylprednisolone and IVIG.
DISCUSSION
Diagnostic Procedures and Results
A skin lesion was positive for varicella-zoster virus (VZV) by polymerase chain reaction ([PCR]; Focus Diagnostics Reference Laboratory, Cypress, CA), and skin and esophageal biopsies performed before transfer were positive for VZV by immunohistochemistry. Quantitative PCR amplified 723 copies/mL VZV from CSF (Focus). Ophthalmologic examination revealed corneal dendrites with features typical of VZV infection. Whole blood and bronchoalveolar lavage fluid were negative for VZV by PCR.
The VZV identified was confirmed to be Oka (vaccine) strain using 4 real-time Forster Resonance Energy Transfer PCR protocols targeting vaccine-associated single nucleotide polymorphisms (Centers for Disease Control and Prevention, Atlanta, GA). In addition, throat swabs obtained 2 months after vaccination were positive for vaccine-genotype mumps and rubella viruses by PCR. Throat swab for measles virus was negative.
Immune evaluation showed profound lymphopenia with markedly low numbers of T cells, B cells, and natural killer cells (Table 1). Although corticosteroid-induced lymphopenia was considered, the striking paucity of lymphocytes prompted concern for primary immunodeficiency. A comprehensive severe combined immunodeficiency (SCID) genetic panel (GeneDx, Gaithersburg, MD) identified compound heterozygous missense mutations in recombination-activating gene 2 (RAG2): a known disease-causing mutation and a novel variant.
Table 1.
Nadir Lymphocyte Counts During Hospitalization
| Cell Type | Marker | Nadir Cell Count, Cells/mm3 (%) | Reference Range, Cells/mm3 (%) |
|---|---|---|---|
| Absolute lymphocyte count | –– | 76 | 1800–9000 |
| T cells | CD3 | 10 (13%) | 2100–6200 (53%–75%) |
| CD4 | 7 (9%) | 1300–3400 (32%–51%) | |
| CD8 | 2 (3%) | 620–2000 (14%–30%) | |
| B cells | CD19 | 51 (67%) | 720–2600 (16%–35%) |
| Natural killer cells | CD16/CD56 | 15 (19%) | 180–920 (3%–15%) |
Treatment and Follow-Up
Due to concern for the development of acyclovir-resistant VZV, foscarnet was added; however, no clinical response was observed. After 8 weeks of illness, she suddenly developed new fever and respiratory distress. During re-intubation, she developed hypotension and bradycardia requiring cardiovascular resuscitation. In the setting of ongoing severe hemolytic anemia, rituximab and plasmapheresis were initiated in an effort to halt autoantibody production and remove previously formed autoantibodies. Over the next 3 days, hepatic and renal failure as well as disseminated intravascular coagulation ensued, support was withdrawn, and the patient died.
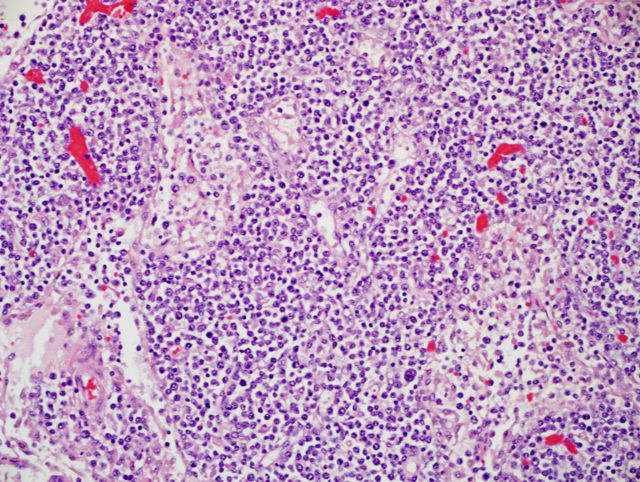
Autopsy revealed severe atrophy of the thymus and lymphoid depletion of the lymph nodes and spleen (Figure 4). Gross examination of the spleen and lungs demonstrated yellow necrotic lesions, forming “puckered” foci of retraction (Figure 5). Multinucleated giant cells were present in the lungs (Figure 6), which stained positive for VZV by immunohistochemistry. Liver specimens also stained weakly positive for VZV. Microscopic examination of the brain showed gliosis of the dorsolateral pons, dorsal medulla, and dorsal horns of the spinal cord consistent with viral effect; however, viral culture, immunohistochemistry (VZV), and PCR (measles, mumps, rubella, and VZV) were negative.
Figure 4.
Post mortem examination of lymph nodes revealing moderate to severe lymphoid depletion with absent follicular architecture. Hematoxylin and eosin stain, 100×.
Figure 5.
Post mortem examination of the lungs demonstrating multifocal scar-like plaques.
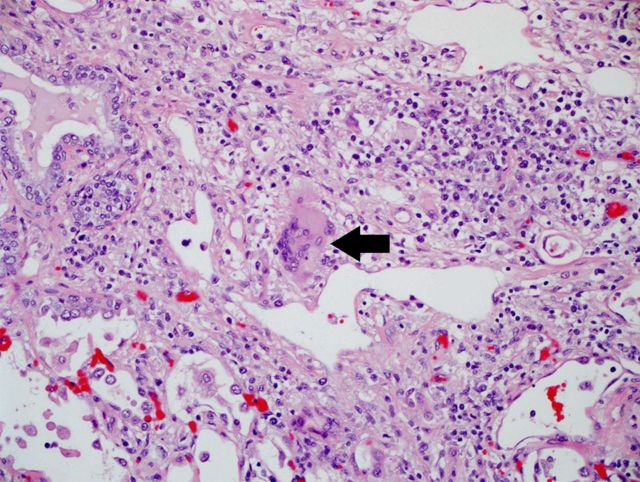
Figure 6.
Post mortem examination of the lungs displaying multinucleated giant cells typical of varicella-zoster virus lesions. Hematoxylin and eosin stain, 100×.
Diagnosis
This patient developed fatal disseminated Oka strain varicella disease, likely due to underlying primary immunodeficiency.
Brief Discussion of Differential and Major Teaching Points of Case
Local or disseminated disease due to Oka strain VZV rarely occurs when the virus reactivates or immediately replicates in a vaccinated patient. Healthy children vaccinated against VZV develop localized herpes zoster at a rate of 48 cases per 100 000 person-years, 46%–60% of which is due to Oka strain [1, 2]. Dissemination of Oka strain VZV to other organs is extremely rare, with fewer than 20 reported cases in children. Immunocompetent individuals can experience dissemination to the central nervous system; however, the presentation is typically mild and recovery is generally complete [3–5]. In immunocompromised patients, disseminated Oka strain VZV can be severe, resulting in chronic or relapsing rash, hepatitis, meningoencephalitis, central nervous system vasculopathy, and/or pneumonitis [6–14].
This is the fourth reported pediatric death associated with Oka strain VZV. The other fatal cases occurred in a 13-month-old female with SCID due to adenosine deaminase deficiency, a 15-month-old female with low T-cell counts but no formal diagnosis of immunodeficiency, and a child receiving chemotherapy for acute lymphoblastic leukemia [15–17]. Severe Oka strain disease has also been described in the setting of various forms of SCID, natural killer T-cell deficiency, DOCK8 mutation, human immunodeficiency virus infection, stem cell transplantation, and treatment with prednisone or chemotherapy [7–14, 18, 19]. In some cases, presentation with disseminated Oka strain VZV led to the diagnosis of a previously unrecognized immunodeficiency [10–14, 17]. This patient demonstrated normal growth and development, tolerated the rotavirus vaccine series, and had no severe or unusual infections before her presentation with Oka strain VZV disease; therefore, immunodeficiency was not suspected before administration of live vaccines at 12 months of age.
The RAG2 mutations identified in this patient offer an explanation for her immunodeficiency. RAG1 and RAG2 encode proteins essential for variability, diversity, and joining (V(D)J) gene segment recombination of T-cell receptors and immunoglobulins [20]. Null mutations in both alleles of either RAG1 or RAG2 result in absence of V(D)J recombination, leading to impaired T- and B-cell development and manifesting as typical SCID. However, a broad range of clinical phenotypes has been described in association with the residual recombination activity that occurs in patients with hypomorphic mutations of RAG1 or RAG2 [21–23]. Autoimmune cytopenias are well described in patients with RAG mutations; thus, this patient's severe hemolytic anemia might be explained by her underlying genetic defects [20, 22, 24, 25]. Alternatively, her autoimmune hemolytic anemia may have been associated with VZV infection, as previously described [26, 27]. Severe VZV infection has been reported in patients with RAG1 or RAG2 deficiency [22]. In addition to underlying immunodeficiency, the use of corticosteroid therapy may have further contributed to the dissemination of Oka strain VZV.
Although the varicella vaccine is contraindicated for most patients with known immunodeficiency, its efficacy and safety profile in immunocompetent children is excellent, and the benefits of the vaccine far outweigh the rare adverse events that can occur in children with undiagnosed immunodeficiency [2, 28]. Newborn screening for T-cell receptor excision circles can enable the early identification of T-cell developmental defects before live virus vaccines are administered. Unfortunately, such screening was not available for this patient at birth.
Acknowledgments
Written informed consent was obtained from the parents to publish photographs of the patient.
Disclaimer. The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the Centers for Disease Control and Prevention, US Department of Health and Human Services.
Potential conflicts of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest.
References
- 1.Weinmann S, Chun C, Schmid DS, et al. Incidence and clinical characteristics of herpes zoster among children in the varicella vaccine era, 2005–2009. J Infect Dis 2013; 208:1859–68. [DOI] [PubMed] [Google Scholar]
- 2.Galea SA, Sweet A, Beninger P, et al. The safety profile of varicella vaccine: a 10-year review. J Infect Dis 2008; 197 (Suppl 2):S165–9. [DOI] [PubMed] [Google Scholar]
- 3.Iyer S, Mittal MK, Hodinka RL. Herpes zoster and meningitis resulting from reactivation of varicella vaccine virus in an immunocompetent child. Ann Emerg Med 2009; 53:792–5. [DOI] [PubMed] [Google Scholar]
- 4.Chouliaras G, Spoulou V, Quinlivan M, et al. Vaccine-associated herpes zoster ophthalmicus and encephalitis in an immunocompetent child. Pediatrics 2010; 125:e969–72. [DOI] [PubMed] [Google Scholar]
- 5.Levin MJ, DeBiasi RL, Bostik V, Schmid DS. Herpes zoster with skin lesions and meningitis caused by 2 different genotypes of the Oka varicella-zoster virus vaccine. J Infect Dis 2008; 198:1444–7. [DOI] [PubMed] [Google Scholar]
- 6.Jean-Philippe P, Freedman A, Chang MW, et al. Severe varicella caused by varicella-vaccine strain in a child with significant T-cell dysfunction. Pediatrics 2007; 120:e1345–9. [DOI] [PubMed] [Google Scholar]
- 7.Bryan CJ, Prichard MN, Daily S, et al. Acyclovir-resistant chronic verrucous vaccine strain varicella in a patient with neuroblastoma. Pediatr Infect Dis J 2008; 27:946–8. [DOI] [PubMed] [Google Scholar]
- 8.Banovic T, Yanilla M, Simmons R, et al. Disseminated varicella infection caused by varicella vaccine strain in a child with low invariant natural killer T cells and diminished CD1d expression. J Infect Dis 2011; 204:1893–901. [DOI] [PubMed] [Google Scholar]
- 9.Levin MJ, Dahl KM, Weinberg A, et al. Development of resistance to acyclovir during chronic infection with the Oka vaccine strain of varicella-zoster virus, in an immunosuppressed child. J Infect Dis 2003; 188:954–9. [DOI] [PubMed] [Google Scholar]
- 10.Kramer JM, LaRussa P, Tsai WC, et al. Disseminated vaccine strain varicella as the acquired immunodeficiency syndrome-defining illness in a previously undiagnosed child. Pediatrics 2001; 108:E39. [DOI] [PubMed] [Google Scholar]
- 11.Ghaffar F, Carrick K, Rogers BB, et al. Disseminated infection with varicella-zoster virus vaccine strain presenting as hepatitis in a child with adenosine deaminase deficiency. Pediatr Infect Dis J 2000; 19:764–6. [DOI] [PubMed] [Google Scholar]
- 12.Levy O, Orange JS, Hibberd P, et al. Disseminated varicella infection due to the vaccine strain of varicella-zoster virus, in a patient with a novel deficiency in natural killer T cells. J Infect Dis 2003; 188:948–53. [DOI] [PubMed] [Google Scholar]
- 13.Bayer DK, Martinez CA, Sorte HS, et al. Vaccine-associated varicella and rubella infections in severe combined immunodeficiency with isolated CD4 lymphocytopenia and mutations in IL7R detected by tandem whole exome sequencing and chromosomal microarray. Clin Exp Immunol 2014; 178:459–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sabry A, Hauk PJ, Jing H, et al. Vaccine strain varicella-zoster virus-induced central nervous system vasculopathy as the presenting feature of DOCK8 deficiency. J Allergy Clin Immunol 2014; 133:1225–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Leung J, Siegel S, Jones JF, et al. Fatal varicella due to the vaccine-strain varicella-zoster virus. Hum Vaccin Immunother 2014; 10:146–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schrauder A, Henke-Gendo C, Seidemann K, et al. Varicella vaccination in a child with acute lymphoblastic leukaemia. Lancet 2007; 369:1232. [DOI] [PubMed] [Google Scholar]
- 17.Woo EJ. Letter to the Editor: Fatal varicella due to the vaccine-strain varicella-zoster virus. Hum Vaccin Immunother 2015; 11:679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chan Y, Smith D, Sadlon T, et al. Herpes zoster due to Oka vaccine strain of varicella zoster virus in an immunosuppressed child post cord blood transplant. J Paediatr Child Health 2007; 43:713–5. [DOI] [PubMed] [Google Scholar]
- 19.Bhalla P, Forrest GN, Gershon M, et al. Disseminated persistent and fatal infection due to the vaccine strain (vOka) of varicella-zoster virus in an adult following stem cell transplantation. Clin Infect Dis 2015; 60:1068–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Niehues T, Perez-Becker R, Schuetz C. More than just SCID–the phenotypic range of combined immunodeficiencies associated with mutations in the recombinase activating genes (RAG) 1 and 2. Clin Immunol 2010; 135:183–92. [DOI] [PubMed] [Google Scholar]
- 21.Villa A, Sobacchi C, Notarangelo LD, et al. V(D)J recombination defects in lymphocytes due to RAG mutations: severe immunodeficiency with a spectrum of clinical presentations. Blood 2001; 97:81–8. [DOI] [PubMed] [Google Scholar]
- 22.Schuetz C, Pannicke U, Jacobsen EM, et al. Lesson from hypomorphic recombination-activating gene (RAG) mutations: why asymptomatic siblings should also be tested. J Allergy Clin Immunol 2014; 133:1211–5. [DOI] [PubMed] [Google Scholar]
- 23.Lee YN, Frugoni F, Dobbs K, et al. A systematic analysis of recombination activity and genotype-phenotype correlation in human recombination-activating gene 1 deficiency. J Allergy Clin Immunol 2014; 133:1099–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen K, Wu W, Mathew D, et al. Autoimmunity due to RAG deficiency and estimated disease incidence in RAG1/2 mutations. J Allergy Clin Immunol 2014; 133:880–2.e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Milner JD, Fasth A, Etzioni A. Autoimmunity in severe combined immunodeficiency (SCID): lessons from patients and experimental models. J Clin Immunol 2008; 28 (Suppl 1):S29–33. [DOI] [PubMed] [Google Scholar]
- 26.Kumar KJ, Kumar HC, Manjunath VG, Arun V. Autoimmune hemolytic anemia due to varicella infection. Iran J Pediatr 2013; 23:491–2. [PMC free article] [PubMed] [Google Scholar]
- 27.Apak H, Karaman S, Dogan Y, et al. Varicella-induced hemolytic anemia with hepatitis. Ann Hematol 2006; 85:64–5. [DOI] [PubMed] [Google Scholar]
- 28.Chaves SS, Haber P, Walton K, et al. Safety of varicella vaccine after licensure in the United States: experience from reports to the vaccine adverse event reporting system, 1995–2005. J Infect Dis 2008; 197 (Suppl 2):S170–7. [DOI] [PubMed] [Google Scholar]