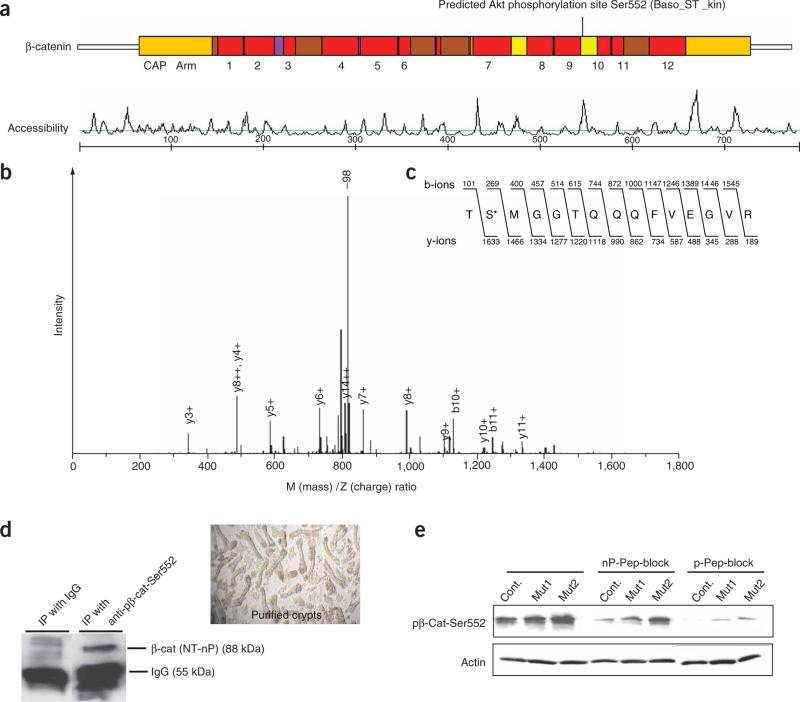
Figure 5.
Identification of the Akt phosphorylation site at the C terminus of β-catenin. (a) Scansite 2.0 output identifying Ser552 as a putative Akt phosphorylation site (basophilic serine-threonine kinase site) at the C terminus of β-catenin. The site is located in the 10th of the 12 armadillo (Arm) repeats and has high accessibility. (b) Mass spectrometry analysis identified a phosphorylated peptide generated by incubation of β-catenin protein with activated Akt. A high-intensity peak corresponding to loss of 98 Da (–98) is indicative of phosphorylation. Additional peaks correspond to the b- and y-ions expected for the peptide shown in c. (c) Amino acid sequence of the β-catenin peptide identified as containing an Akt phosphorylation site, showing the masses of the expected b- and y-ions. The asterisk marks the phosphorylated Ser552. (d) Confirmation that anti-p-β-cat-Ser552, recognizes the active form of β-catenin. Immunoprecipitation (IP) was performed using anti-p-β-cat-Ser552 or IgG as a control and subsequently blotted using an antibody to N-terminally nonphosphorylated β-catenin (NT-nP) from purified crypts. Image at right shows purified crypts used for the IP assay. (e) Protein blot determination of the specificity of anti-p-β-cat-Ser552 using nonphosphorylated (nP) and phosphorylated (p) peptide block.