Fig. 3.
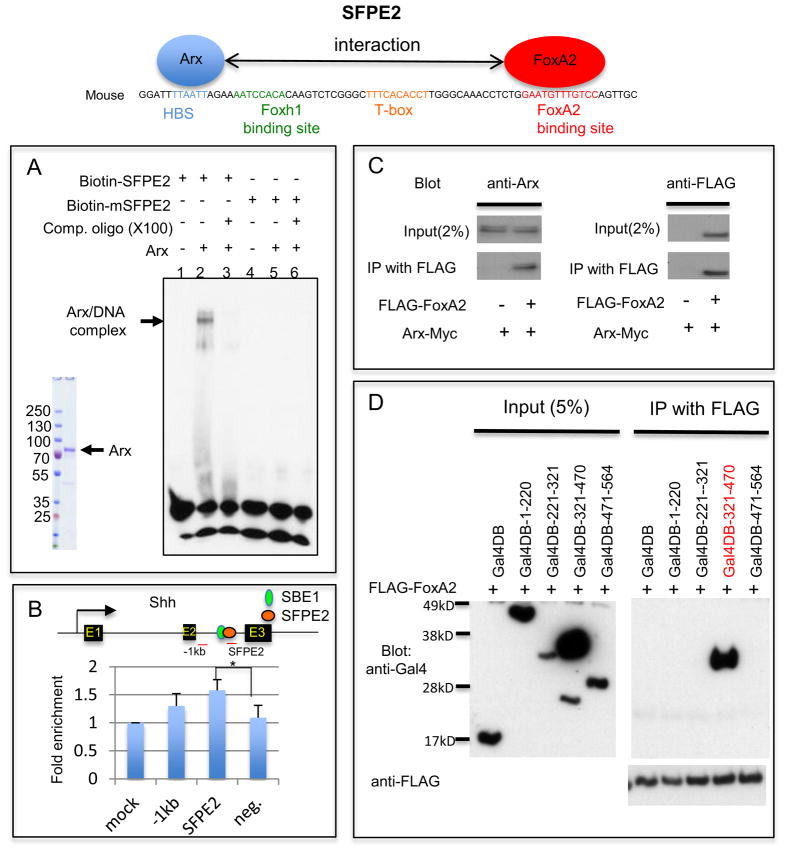
Arx binds to the HBS within Shh enhancer (SFPE2) and interacts with FoxA2. A) EMSA with the full-length Arx protein purified from transfected HEK293T cells and SFPE2 DNA fragments shows the DNA/protein complex (lane 2). The DNA/protein complex was reduced in the presence of unlabeled SFPE2 DNA competitor (lane 3). However, when the SFPE2 is mutated in the HBS (mSFPE2), it can no longer bind to Arx (lane 4–6). B) ChIP assay using developing spinal cord with either an anti-Arx antibody or control IgG. Enrichment of the PCR product in the immmunoprecipitated sample with Anti-Arx antibody was observed with three primer sets (one 1kb upstream region of SFPE2, a second in SFPE2 and the third a negative control which is in GAPDH locus) compared to control. (Note: ChIP was performed on whole spinal cords, of which less than 0.01% is FP.) Error bars correspond to SD (*, p=0.04, two-tailed, unpaired t-test). C) Arx directly interacts with FoxA2. HEK293T cells transfected with Arx-Myc and FoxA2-FLAG were used for immunoprecipitation (IP) experiments. IP with FLAG (FoxA2) antibody confirmed an interaction with Arx (Western blot with anti-Arx antibody). D) Arx interacts with FoxA2 through its homeodomain. HEK293T cells transfected with a series of Arx deletion mutants (aa 1–220, 221–321, 321–470 and 471–564 conjugated to Gal4DB) with FLAG-FoxA2, were used for IP. An Arx construct containing the homeodomain, Gal4DB-321-470 (red), was co-immunoprecipitated with FoxA2, whereas other constructs were not. The arrows identify the full-length protein of each mutant.