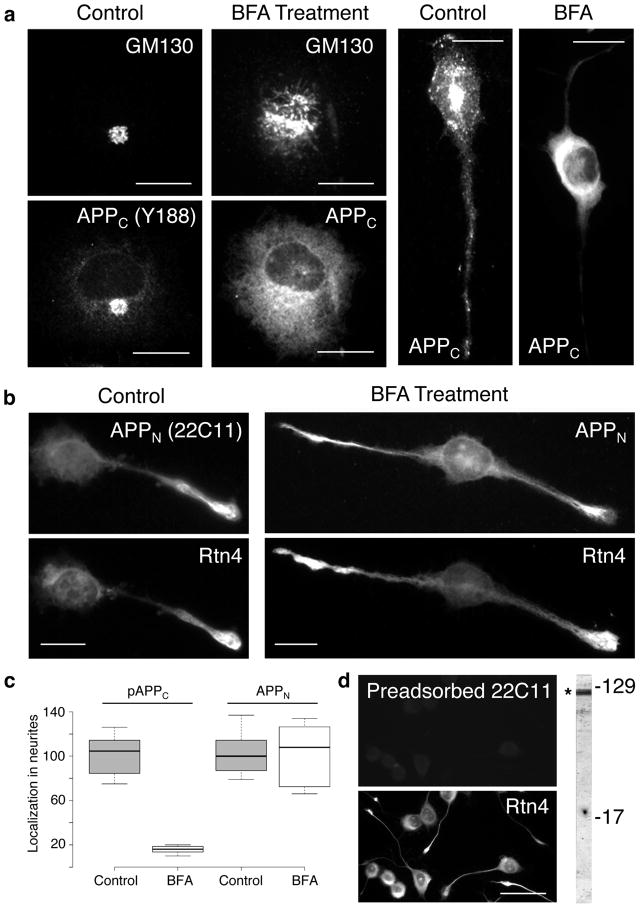
Figure 1.
Transport into neurites of sAPP is not affected by Brefeldin A (BFA) treatment. CAD cells were subjected to BFA treatment for 22 hrs, and probed for the distribution of GM130 (a Golgi marker), APP C-terminal (APPC, detected with antibody Y188; a) and N-terminal (APPN, detected with antibody 22C11; b) epitopes, and Rtn4 (b). Controls without BFA treatment are also shown. The box plot in (c) shows that BFA treatment blocks translocation of phosphorylated APPC (pAPPC), but not of APPN into neurites. Translocation of nonphosphorylated APPC is also blocked by BFA treatment. (d) Control, dual labeling experiment, showing specificity of the 22C11 antibody for APP species. Antibody 22C11 was used after preadsorption on APP, a procedure that removed the anti-APP immunoreactive species from the IgG fraction (see Methods). Preadsorption on BSA revealed the typical immunolabeling with antibody 22C11, similar to that seen in (b) (not shown). The blot at right shows that antibody 22C11 detects polypeptides in the region of APP/sAPP (*). In a previous study, we showed that sAPPβ migrates within this region of the blot, and represents a substantial fraction of the immunolabeled band [16]. Molecular weight markers are given in kilodaltons. Bars = 20 μm (a and b); 50 μm (d).